Sensitive detection of the IS 6110 sequence of Mycobacterium tuberculosis complex based on PCR-magnetic bead ELISA
- PMID: 35548803
- PMCID: PMC9086544
- DOI: 10.1039/c8ra06599c
Sensitive detection of the IS 6110 sequence of Mycobacterium tuberculosis complex based on PCR-magnetic bead ELISA
Abstract
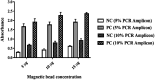
Tuberculosis (TB) is ranked as the top killer among infectious diseases worldwide. Early and accurate diagnosis of the disease is crucial to end the global TB epidemic. The current commercially available molecular tests are still unaffordable by most TB affected communities. Herein, we developed a novel rapid and sensitive diagnostic method to detect the IS6110 sequence of Mycobacterium tuberculosis (M. tuberculosis) complex using PCR-magnetic bead ELISA. PCR amplification ofa 123 bp repetitive sequence of the IS6110 gene was performed by using digoxigenin (DIG) and biotin-labelled primers. Streptavidin-conjugated magnetic beads were used to collect the dual-labelled amplicons and subsequently, colourimetric detection was done by using horseradish peroxidase (HRP)-conjugated anti-DIG antibody. This method is able to detect M. tuberculosis DNA down to 0.5 fg per reaction within 3 hours. The sensitivity of IS6110 PCR detection by magnetic bead ELISA is 100 times higher than that of conventional agarose gel electrophoresis. The assay specificity was determined using a panel of DNA extracted from 10 common bacteria causing lower respiratory tract infections. No cross-reactivity was detected from those bacteria by IS6110 PCR-magnetic bead ELISA. Thus, the novel highly sensitive and specific, reduced assay time and simplicity of this PCR-magnetic bead ELISA for the detection of the specific gene of M. tuberculosis complex makes it an attractive diagnostic tool for large-scale screening of tuberculosis in standard clinical laboratories.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Detection of Mycobacterium tuberculosis in clinical samples using insertion sequences IS6110 and IS990.Tuberculosis (Edinb). 2001;81(4):271-8. doi: 10.1054/tube.2001.0301. Tuberculosis (Edinb). 2001. PMID: 11584595
-
Evaluation of digital PCR assay in detection of M.tuberculosis IS6110 and IS1081 in tuberculosis patients plasma.BMC Infect Dis. 2020 Sep 7;20(1):657. doi: 10.1186/s12879-020-05375-y. BMC Infect Dis. 2020. PMID: 32894079 Free PMC article.
-
Two target genes based multiple cross displacement amplification combined with a lateral flow biosensor for the detection of Mycobacterium tuberculosis complex.BMC Microbiol. 2021 Oct 4;21(1):267. doi: 10.1186/s12866-021-02328-6. BMC Microbiol. 2021. PMID: 34607556 Free PMC article.
-
[New era in molecular epidemiology of tuberculosis in Japan].Kekkaku. 2006 Nov;81(11):693-707. Kekkaku. 2006. PMID: 17154049 Review. Japanese.
-
[Rapid detection and identification of mycobacteria by the PCR assay based on the co-amplification of the gene IS6110 and groEL].Rinsho Byori. 1995 Oct;43(10):1051-6. Rinsho Byori. 1995. PMID: 8531389 Review. Japanese.
Cited by
-
Evaluating the Sensitivity of Different Molecular Techniques for Detecting Mycobacterium tuberculosis Complex in Patients with Pulmonary Infection.Pol J Microbiol. 2023 Dec 16;72(4):421-431. doi: 10.33073/pjm-2023-040. eCollection 2023 Dec 1. Pol J Microbiol. 2023. PMID: 37934050 Free PMC article.
-
Nanoparticles as an Alternative Strategy for the Rapid Detection of Mycobacterium tuberculosis Complex (MTBC): A Systematic Literature Review of In Vitro Studies.IET Nanobiotechnol. 2025 May 29;2025:4639233. doi: 10.1049/nbt2/4639233. eCollection 2025. IET Nanobiotechnol. 2025. PMID: 40476137 Free PMC article.
-
Advances on Electrochemiluminescent Biosensors for TB Biomarkers.ACS Sens. 2025 Apr 25;10(4):2409-2430. doi: 10.1021/acssensors.4c03517. Epub 2025 Apr 9. ACS Sens. 2025. PMID: 40202785 Free PMC article. Review.
-
Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends.Pharmaceutics. 2021 Jun 24;13(7):943. doi: 10.3390/pharmaceutics13070943. Pharmaceutics. 2021. PMID: 34202604 Free PMC article. Review.
References
-
- World Health Organization, in Global tuberculosis report 2017, 2017
-
- World Health Organization, Bending the curve—ending TB: annual report 2017, WHO report, 2017
-
- García-Basteiro A. L. DiNardo A. Saavedra B. et al., Point of care diagnostics for tuberculosis. Rev. Port. Pneumol. 2018 - PubMed
-
- Boehme C. C. Nicol M. P. Nabeta P. et al., Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377(9776):1495–1505. doi: 10.1016/S0140-6736(11)60438-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

