A highly sensitive and selective chemosensor for Pb2+ based on quinoline-coumarin
- PMID: 35548814
- PMCID: PMC9086682
- DOI: 10.1039/c8ra04935a
A highly sensitive and selective chemosensor for Pb2+ based on quinoline-coumarin
Abstract
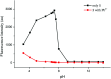
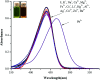
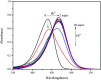
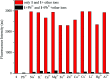
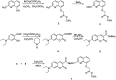
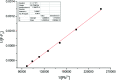
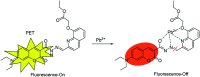
In this study, a highly sensitive and selective fluorescent chemosensor, ethyl(E)-2-((2-((2-(7-(diethylamino)-2-oxo-2H-chromene-3-carbonyl)hydrazono)methyl)quinolin-8-yl)oxy)acetate (1), was synthesized and characterized by 1H NMR, 13C NMR and ESI-MS. Sensor 1 showed an "on-off" fluorescence response to Pb2+ with a 1 : 1 binding stoichiometry in CH3CN/HEPES buffer medium (9 : 1 v/v). The detection limit of sensor 1 to Pb2+ was determined to be 0.5 μM, and the stable pH range for Pb2+ detection was from 4 to 8.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
A novel fluorescent chemosensor based on coumarin and quinolinyl-benzothiazole for sequential recognition of Cu2+ and PPi and its applicability in live cell imaging.Spectrochim Acta A Mol Biomol Spectrosc. 2020 Apr 5;230:118022. doi: 10.1016/j.saa.2019.118022. Epub 2020 Jan 3. Spectrochim Acta A Mol Biomol Spectrosc. 2020. PMID: 31927510
-
A New Fluorescent "Turn-Off" Coumarin-Based Chemosensor: Synthesis, Structure and Cu-Selective Fluorescent Sensing in Water Samples.J Fluoresc. 2017 Jul;27(4):1293-1298. doi: 10.1007/s10895-017-2062-x. Epub 2017 Mar 10. J Fluoresc. 2017. PMID: 28283898
-
A novel fluorescent sensor for specific recognition of GSH based on the copper complex and its bioimaging in living cells.Bioorg Chem. 2020 Jul;100:103923. doi: 10.1016/j.bioorg.2020.103923. Epub 2020 May 11. Bioorg Chem. 2020. PMID: 32417525
-
A chemosensor with a paddle structure based on a BODIPY chromophore for sequential recognition of Cu2+ and HSO3.RSC Adv. 2019 Oct 28;9(59):34652-34657. doi: 10.1039/c9ra08345f. eCollection 2019 Oct 23. RSC Adv. 2019. PMID: 35530010 Free PMC article.
-
Highly selective and sensitive coumarin-triazole-based fluorometric 'turn-off' sensor for detection of Pb2+ ions.Luminescence. 2018 Jun;33(4):713-721. doi: 10.1002/bio.3468. Epub 2018 Mar 2. Luminescence. 2018. PMID: 29498808
Cited by
-
Dual Colorimetric Sensor for Hg2+/Pb2+ and an Efficient Catalyst Based on Silver Nanoparticles Mediating by the Root Extract of Bistorta amplexicaulis.Front Chem. 2020 Oct 22;8:591958. doi: 10.3389/fchem.2020.591958. eCollection 2020. Front Chem. 2020. PMID: 33195096 Free PMC article.
-
A Colorimetric Distinct Color Change Cu(II) 4-{[1-(2,5-dihydroxyphenyl)ethylidene]amino}-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one Chemosensor and its Application as a Paper Test Kit.J Fluoresc. 2023 May;33(3):1089-1099. doi: 10.1007/s10895-022-03034-w. Epub 2022 Dec 27. J Fluoresc. 2023. PMID: 36574186
-
A coumarin-based fluorescent chemosensor as a Sn indicator and a fluorescent cellular imaging agent.RSC Adv. 2023 Mar 27;13(15):9811-9823. doi: 10.1039/d2ra07884h. eCollection 2023 Mar 27. RSC Adv. 2023. PMID: 36994144 Free PMC article.
-
Nitroquinolone Fused Salicyl and Naphthyl Hydrazone Fluorescent Probes for the Detection of Fe3+and Pb2+ Ions.J Fluoresc. 2025 Jun;35(6):4129-4142. doi: 10.1007/s10895-024-03813-7. Epub 2024 Jul 2. J Fluoresc. 2025. PMID: 38954084
-
Mo(vi) complexes of amide-imine conjugates for tuning the selectivity of fluorescence recognition of Y(iii) vs. Pb(ii).RSC Adv. 2022 Nov 21;12(51):33293-33303. doi: 10.1039/d2ra06035c. eCollection 2022 Nov 15. RSC Adv. 2022. PMID: 36425161 Free PMC article.
References
-
- Zhang Z. Q. He X. W. Shang Y. J. Yu Z. Y. Wang S. F. Wu F. L. Chem. Res. Chin. Univ. 2017;33:31–35. doi: 10.1007/s40242-017-6239-2. - DOI
-
- Goswami S. Manna A. Aich K. Paul S. Chem. Lett. 2012;41:1600–1602. doi: 10.1246/cl.2012.1600. - DOI
-
- Sunnapu O. Kotla N. G. Maddiboyina B. Singaravadivel S. Sivaraman G. RSC Adv. 2016;6:656–660. doi: 10.1039/C5RA20482H. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

