Investigation of dynamical properties of methane in slit-like quartz pores using molecular simulation
- PMID: 35548817
- PMCID: PMC9086685
- DOI: 10.1039/c8ra06678g
Investigation of dynamical properties of methane in slit-like quartz pores using molecular simulation
Abstract
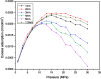
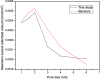
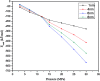
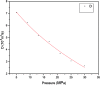
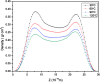
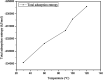
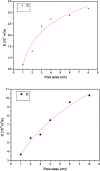
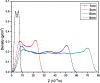
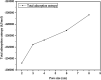
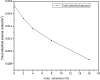
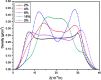
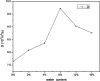
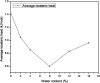
The dynamical properties of adsorption media confined in micropores play an important role in the adsorptive separation of fluids. However, a problem is that it is difficult to directly use approaches based on experimental measurements. Molecular simulation has been an effective tool for investigating the diffusion of fluids on the microscale in recent years. In this work, the diffusion properties of methane in quartz were mainly investigated from a microscale viewpoint using MD (molecular dynamics) methods, and this paper primarily discusses the influence of parameters such as pressure, temperature, pore size and water content on the diffusion and thermodynamic parameters of methane in slit-like quartz pores. The results demonstrate that the transport ability of quartz pores decreases with an increase in pressure in pores of a fixed size at a certain temperature and increases with an increase in pore size or temperature at a fixed pressure, which is related to changes in the interaction between methane molecules and quartz. In the pressure range used in the simulation, the average isosteric heat of adsorption of methane increases with an increase in pressure and is in the range of 6.52-10.794 kJ mol-1. Therefore, the gas adsorption behavior is classed as physical adsorption because the heat of adsorption is significantly lower than the minimum heat of gas adsorption for chemisorption. The increase in the total adsorption entropy is caused by an increase in temperature due to an increase in internal energy, which brings about a reduction in the interactions between gas molecules and walls of quartz. However, with an increase in pore size the total adsorption entropy increases, for which an explanation may be that in pores of a larger size methane molecules are adsorbed at higher-energy sites and generate a higher isosteric heat, which causes a reduction in interactions between the adsorbate and adsorbent. Regarding the influence of different water contents on the diffusion of methane, it was demonstrated that with an increase in moisture the mobility of methane molecules initially increases and then decreases, which is related to the distance between gas molecules.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Molecular insight into the diffusion/flow potential properties initiated by methane adsorption in coal matrix: taking the factor of moisture contents into account.Environ Sci Pollut Res Int. 2022 May;29(24):36225-36242. doi: 10.1007/s11356-022-18704-2. Epub 2022 Jan 21. Environ Sci Pollut Res Int. 2022. PMID: 35061177
-
Heat of adsorption, adsorption stress, and optimal storage of methane in slit and cylindrical carbon pores predicted by classical density functional theory.Phys Chem Chem Phys. 2018 Jan 3;20(2):872-888. doi: 10.1039/c7cp06591d. Phys Chem Chem Phys. 2018. PMID: 29239426
-
Examination of hydrophobic contaminant adsorption in mineral micropores with grand canonical Monte Carlo simulations.Environ Sci Technol. 2003 May 1;37(9):1775-82. doi: 10.1021/es0260014. Environ Sci Technol. 2003. PMID: 12775048
-
Adsorption/desorption characteristics of methane on moist shale under high temperature and pressure: an experimental and molecular simulation study.Environ Technol. 2025 Apr;46(10):1679-1692. doi: 10.1080/09593330.2024.2398810. Epub 2024 Sep 9. Environ Technol. 2025. PMID: 39250824
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
References
-
- EIA, International Energy Outlook 2017 Overview, U.S. Energy Inf. Adm., 2017, vol. IEO2017, no. 2017, p. 143
-
- Curtis J. B. Fractured shale-gas systems. Am. Assoc. Pet. Geol. Bull. 2002;86(11):1921–1938.
-
- Ji L. Zhang T. Milliken K. L. Qu J. Zhang X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012;27(12):2533–2545. doi: 10.1016/j.apgeochem.2012.08.027. - DOI
-
- Yingjie L. Xiaoyuan L. Yuelong W. Qingchun Y. Effects of composition and pore structure on the reservoir gas capacity of Carboniferous shale from Qaidam Basin, China. Mar. Pet. Geol. 2015;62:44–57. doi: 10.1016/j.marpetgeo.2015.01.011. - DOI
-
- Liu F. L. X. Xiong F. L. J. Liang F. L. L. Journal of Natural Gas Science and Engineering Investigation of pore structure and fractal characteristics of organic- rich Yanchang formation shale in central China by nitrogen adsorption/desorption analysis. J. Nat. Gas Sci. Eng. 2015;22(April 2011):62–72. doi: 10.1016/j.jngse.2014.11.020. - DOI
LinkOut - more resources
Full Text Sources

