Molecular insights into competitive adsorption of CO2/CH4 mixture in shale nanopores
- PMID: 35548842
- PMCID: PMC9086684
- DOI: 10.1039/c8ra07486k
Molecular insights into competitive adsorption of CO2/CH4 mixture in shale nanopores
Abstract
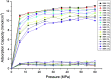
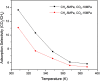
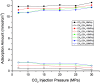
In the present study, competitive adsorption behaviour of supercritical carbon dioxide and methane binary mixture in shale organic nanopores was investigated by using grand canonical Monte Carlo (GCMC) simulations. The model was firstly validated by comparing with experimental data and a satisfactory agreement was obtained. Then the effects of temperature (298-388 K), pressure (up to 60 MPa), pore size (1-4 nm) and moisture content (0-2.4 wt%) on competitive adsorption behaviour of the binary mixture were examined and discussed in depth. It is found that the adsorption capacity of carbon dioxide in shale organic nanopores is much higher than that of methane under various conditions. The mechanism of competitive adsorption was discussed in detail. In addition, the results show that a lower temperature is favorable to both the adsorption amount and selectivity of CO2/CH4 binary mixture in shale organic nanopores. However, an appropriate CO2 injection pressure should be considered to take into account the CO2 sequestration amount and the exploitation efficiency of shale gas. As for moisture content, different influences on CO2/CH4 adsorption selectivity have been observed at low and high moisture conditions. Therefore, different simulation technologies for shale gas production and CO2 sequestration should be applied depending on the actual moisture conditions of the shale reservoirs. It is expected that the findings in this work could be helpful to estimate and enhance shale gas resource recovery and also evaluate CO2 sequestration efficiency in shale reservoirs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures











References
-
- Yang Y. Wang L. Fang Y. Mou C. Renewable Sustainable Energy Rev. 2016;76:1465–1478. doi: 10.1016/j.rser.2016.11.174. - DOI
-
- Wang L. Wang S. Zhang R. Wang C. Xiong Y. Zheng X. Li S. Jin K. Rui Z. J. Nat. Gas Sci. Eng. 2017;37:560–578. doi: 10.1016/j.jngse.2016.11.051. - DOI
-
- Guo M. Lu X. Nielsen C. P. McElroy M. B. Shi W. Chen Y. Xu Y. Renewable Sustainable Energy Rev. 2016;66:742–750. doi: 10.1016/j.rser.2016.08.026. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

