New 1,2,3-triazole linked ciprofloxacin-chalcones induce DNA damage by inhibiting human topoisomerase I& II and tubulin polymerization
- PMID: 35548854
- PMCID: PMC9116245
- DOI: 10.1080/14756366.2022.2072308
New 1,2,3-triazole linked ciprofloxacin-chalcones induce DNA damage by inhibiting human topoisomerase I& II and tubulin polymerization
Abstract
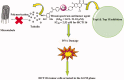
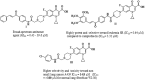
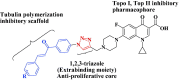
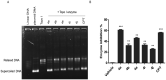
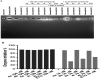
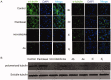
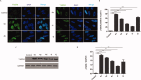
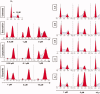
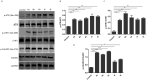
A series of novel 1,2,3-triazole-linked ciprofloxacin-chalcones 4a-j were synthesised as potential anticancer agents. Hybrids 4a-j exhibited remarkable anti-proliferative activity against colon cancer cells. Compounds 4a-j displayed IC50s ranged from 2.53-8.67 µM, 8.67-62.47 µM, and 4.19-24.37 µM for HCT116, HT29, and Caco-2 cells; respectively, whereas the doxorubicin, showed IC50 values of 1.22, 0.88, and 4.15 µM. Compounds 4a, 4b, 4e, 4i, and 4j were the most potent against HCT116 with IC50 values of 3.57, 4.81, 4.32, 4.87, and 2.53 µM, respectively, compared to doxorubicin (IC50 = 1.22 µM). Also, hybrids 4a, 4b, 4e, 4i, and 4j exhibited remarkable inhibitory activities against topoisomerase I, II, and tubulin polymerisation. They increased the protein expression level of γH2AX, indicating DNA damage, and arrested HCT116 in G2/M phase, possibly through the ATR/CHK1/Cdc25C pathway. Thus, the novel ciprofloxacin hybrids could be exploited as potential leads for further investigation as novel anticancer medicines to fight colorectal carcinoma.
Keywords: Click synthesis; DNA damage; HCT116 cells; chalcone; ciprofloxacin; cytotoxicity; topoisomerase I and II inhibitors; tubulin polymerisation inhibition.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Abdel-Aal MAA, Abdel-Aziz SA, Shaykoon MSA, Abuo-Rahma GE-DA.. Towards anticancer fluoroquinolones: a review article. Arch Pharm 2019;352:e1800376. - PubMed
-
- Kaushik CP, Sangwan J, Luxmi R, et al. . Design, synthesis, anticancer and antioxidant activities of amide linked 1,4-disubstituted 1,2,3-triazoles. J. Mol. Struct 2021;1226:129255.
-
- Chekkara R, Gorla VR, Susithra E, et al. . Design, synthesis and anticancer evaluation of 2-amino pyrimidine linked 7-azaindzole derivatives. Chem. Data Collect 2020;29:100513.
-
- El-Damasy AK, Haque MM, Park JW, et al. . 2-Anilinoquinoline based arylamides as broad spectrum anticancer agents with B-RAFV600E/C-RAF kinase inhibitory effects: design, synthesis, in vitro cell-based and oncogenic kinase assessments. Eur J Med Chem 2020;208:112756. - PubMed
-
- Baglini E, Salerno S, Barresi E, et al. . Multiple topoisomerase I (TopoI), topoisomerase II (TopoII) and Tyrosyl-DNA phosphodiesterase (TDP) inhibitors in the development of anticancer drugs. Eur J Pharm Sci 2021;156:105594. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
