δ-C-H Halogenation Reactions Enabled by a Nitrogen-Centered Radical Precursor
- PMID: 35549294
- PMCID: PMC10292861
- DOI: 10.1021/acs.orglett.2c01261
δ-C-H Halogenation Reactions Enabled by a Nitrogen-Centered Radical Precursor
Abstract
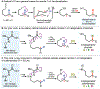
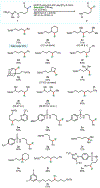
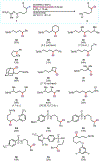
Nitrogen-centered radicals are versatile synthetic intermediates with the ability to undergo diverse reactions such as hydrogen atom transfer (HAT), β-scission, and addition across unsaturated systems. A long-standing impediment to the wider adoption of these intermediates in synthesis has been the difficulty of their generation. Herein we disclose a new hydrazonyl carboxylic acid precursor to nitrogen-centered radicals and its application toward remote C-H fluorination and chlorination reactions of sulfonyl-protected alkyl amines via 1,5-HAT.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
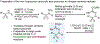
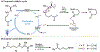
References
-
- Xu J-W; Zhang Z-Z; Rao W-H; Shi B-F Site-Selective Alkenylation of δ-C(sp3)–H Bonds with Alkynes via a Six-Membered Palladacycle. J. Am. Chem. Soc 2016, 138, 10750–10753. - PubMed
-
- Xu Y; Young MC; Wang C; Magness DM; Dong G Catalytic C(sp3)−H Arylation of Free Primary Amines with an exo Directing Group Generated in situ. Angew. Chem. Int. Ed 2016, 55, 9084–9087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

