Head and Neck Paragangliomas: An Update on the Molecular Classification, State-of-the-Art Imaging, and Management Recommendations
- PMID: 35549357
- PMCID: PMC9152685
- DOI: 10.1148/rycan.210088
Head and Neck Paragangliomas: An Update on the Molecular Classification, State-of-the-Art Imaging, and Management Recommendations
Abstract
Paragangliomas are neuroendocrine tumors that derive from paraganglia of the autonomic nervous system, with the majority of parasympathetic paragangliomas arising in the head and neck. More than one-third of all paragangliomas are hereditary, reflecting the strong genetic predisposition of these tumors. The molecular basis of paragangliomas has been investigated extensively in the past couple of decades, leading to the discovery of several molecular clusters and more than 20 well-characterized driver genes (somatic and hereditary), which are more than are known for any other endocrine tumor. Head and neck paragangliomas are largely related to the pseudohypoxia cluster and have been previously excluded from most molecular profiling studies. This review article introduces the molecular classification of paragangliomas, with a focus on head and neck paragangliomas, and discusses its impact on the management of these tumors. Genetic testing is now recommended for all patients with paragangliomas to provide screening and surveillance recommendations for patients and relatives. While CT and MRI provide excellent anatomic characterization of paragangliomas, gallium 68 tetraazacyclododecane tetraacetic acid-octreotate (ie, 68Ga-DOTATATE) has superior sensitivity and is recommended as first-line imaging in patients with head and neck paragangliomas with concern for multifocal and metastatic disease, patients with known multifocal and metastatic disease, and in candidates for targeted peptide-receptor therapy. Keywords: Molecular Imaging, MR Perfusion, MR Spectroscopy, Neuro-Oncology, PET/CT, SPECT/CT, Head/Neck, Genetic Defects © RSNA, 2022.
Keywords: Genetic Defects; Head/Neck; MR Perfusion; MR Spectroscopy; Molecular Imaging; Neuro-Oncology; PET/CT; SPECT/CT.
Conflict of interest statement
Figures
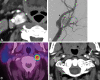
![Histopathologic analysis. (A, B) Type I chief cells in well-defined
nests (* = zellballen) surrounded by type II sustentacular cells
(arrows). (Hematoxylin-eosin stain; original magnification, [A] ×10
and [B] ×40.) (C) Type I chief cells (*). (Synaptophysin
stain; original magnification, ×10.) (D) Type II sustentacular cells
(arrow). (S100 stain; original magnification, ×10.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ec61/9152685/123e37078225/rycan.210088.fig2.gif)




References
-
- Beard CM , Sheps SG , Kurland LT , Carney JA , Lie JT . Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979 . Mayo Clin Proc 1983. ; 58 ( 12 ): 802 – 804 . - PubMed
-
- Stenström G , Svärdsudd K . Pheochromocytoma in Sweden 1958-1981. An analysis of the National Cancer Registry Data . Acta Med Scand 1986. ; 220 ( 3 ): 225 – 232 . - PubMed
-
- Favier J , Amar L , Gimenez-Roqueplo AP . Paraganglioma and phaeochromocytoma: from genetics to personalized medicine . Nat Rev Endocrinol 2015. ; 11 ( 2 ): 101 – 111 . - PubMed
-
- Lenders JW , Duh QY , Eisenhofer G , et al. . Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline . J Clin Endocrinol Metab 2014. ; 99 ( 6 ): 1915 – 1942 . - PubMed
-
- Castro-Vega LJ , Lepoutre-Lussey C , Gimenez-Roqueplo AP , Favier J . Rethinking pheochromocytomas and paragangliomas from a genomic perspective . Oncogene 2016. ; 35 ( 9 ): 1080 – 1089 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

