Gaze following requires early visual experience
- PMID: 35549552
- PMCID: PMC9171757
- DOI: 10.1073/pnas.2117184119
Gaze following requires early visual experience
Abstract
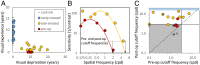
Gaze understanding—a suggested precursor for understanding others’ intentions—requires recovery of gaze direction from the observed person's head and eye position. This challenging computation is naturally acquired at infancy without explicit external guidance, but can it be learned later if vision is extremely poor throughout early childhood? We addressed this question by studying gaze following in Ethiopian patients with early bilateral congenital cataracts diagnosed and treated by us only at late childhood. This sight restoration provided a unique opportunity to directly address basic issues on the roles of “nature” and “nurture” in development, as it caused a selective perturbation to the natural process, eliminating some gaze-direction cues while leaving others still available. Following surgery, the patients’ visual acuity typically improved substantially, allowing discrimination of pupil position in the eye. Yet, the patients failed to show eye gaze-following effects and fixated less than controls on the eyes—two spontaneous behaviors typically seen in controls. Our model for unsupervised learning of gaze direction explains how head-based gaze following can develop under severe image blur, resembling preoperative conditions. It also suggests why, despite acquiring sufficient resolution to extract eye position, automatic eye gaze following is not established after surgery due to lack of detailed early visual experience. We suggest that visual skills acquired in infancy in an unsupervised manner will be difficult or impossible to acquire when internal guidance is no longer available, even when sufficient image resolution for the task is restored. This creates fundamental barriers to spontaneous vision recovery following prolonged deprivation in early age.
Keywords: blind; cataract; gaze; joint attention; vision.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Brooks R., Meltzoff A. N., “Gaze following: A mechanism for building social connections between infants and adults” in Mechanisms of Social Connection: From Brain to Group, Mikulincer M., Shaver P. R., Eds. (American Psychological Association, 2013), pp. 167–183.
-
- Moore C., Corkum V., Social understanding at the end of the first year of life. Dev. Rev. 14, 349–372 (1994).
-
- Suzuki M., Izawa A., Takahashi K., Yamazaki Y., The coordination of eye, head, and arm movements during rapid gaze orienting and arm pointing. Exp. Brain Res. 184, 579–585 (2008). - PubMed
-
- Tomasello M., Hare B., Lehmann H., Call J., Reliance on head versus eyes in the gaze following of great apes and human infants: The cooperative eye hypothesis. J. Hum. Evol. 52, 314–320 (2007). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

