Nup50 plays more than one instrument
- PMID: 35549614
- PMCID: PMC9359400
- DOI: 10.1080/15384101.2022.2074742
Nup50 plays more than one instrument
Abstract
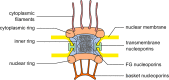
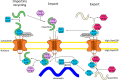
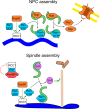
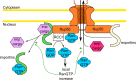
Nup50 is nuclear pore complex component localized to the nuclear side of the pore and in the nucleoplasm. It has been characterized as an auxiliary factor in nuclear transport reactions. Our recent work indicates that it interacts with and stimulates RCC1, the sole guanine nucleotide exchange factor for the GTPase Ran. Here, we discuss how this interaction might contribute to Nup50 function in nuclear transport but also its other functions like control of gene expression, cell cycle and DNA damage repair.
Keywords: DNA repair; Nup50; RCC1; cell cycle; nuclear pore assembly; nuclear trafficking; p27Kip1.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
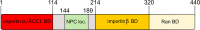
References
-
- Kutay U, Juhlen R, Antonin W.. Mitotic disassembly and reassembly of nuclear pore complexes. Trends Cell Biol. 2021;31(12):1019–1033. - PubMed
-
- Fan F, Liu CP, Korobova O, et al. cDNA cloning and characterization of Npap60: a novel rat nuclear pore-associated protein with an unusual subcellular localization during male germ cell differentiation. Genomics. 1997;40(3):444–453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
