The Wider Considerations in Closing Chronic Disease Gaps - Focus on Heart Failure and Implementation
- PMID: 35549873
- PMCID: PMC10201899
- DOI: 10.2174/1573403X18666220512160737
The Wider Considerations in Closing Chronic Disease Gaps - Focus on Heart Failure and Implementation
Abstract
Background: Heart failure (HF) is predominately a chronic disease. There are overlaps in HF and chronic disease research and care. Chronic disease and HF research are conducted with multiple goals. The overarching goal is "optimized patient outcomes at maximum costeffectiveness". However, observations on patients can come with many variables; thus, we see differences in clinical translation. This document discusses an argument for three important gaps common to HF and chronic disease, i.e., screening, self-management, and patient-reported outcomes (PRO), and provides a glance of how it could fit into the evidence tree. Pertinent arguments for a framework for health services and models of care are provided as a prelude to future consensus.
Methodology: 1) A preliminary literature review to identify a taxonomy for cardiovascular research, and 2) a review of the published literature describing the translation of research studies into clinical practice for cardiovascular disorders. A spectrum from observational to large randomized controlled trials to post-marketing studies were identified.
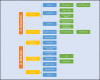
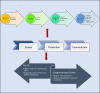
Discussion: A brief discussion on traditional research and differences focusing on screening, mixed methods research concepts, and chronic diseases models of care. Six steps to facilitate this: 1) Research design; 2) Research application (translation) i. routine ii. challenges; 3. Transforming research to translational level; 4. Funding and infrastructure; 5. Clinical Centres of Research Excellence (CCRE) and collaboration; 6. Governance and cost-effectiveness.
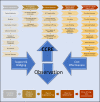
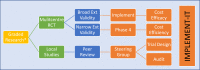
Conclusion: Implementation research that aims to link research findings to improved patient outcomes in an efficient and effective way is a neglected area. Skills required to perform implementation research are complex. Ways to maximize translational impacts for chronic disease research to clinical practice are described in a HF context.
Keywords: Chronic disease; clinical translation; health policy; heart failure; patient-reported outcomes; screening.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
All co-authors have won independent and governmental research funding. None pose a conflict of interest for this review.
Figures

References
-
- Krumholz H.M., Currie P.M., Riegel B., et al. American Heart Association Disease Management Taxonomy Writing Group. A taxonomy for disease management: A scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006;114(13):1432–1445. doi: 10.1161/CIRCULATIONAHA.106.177322. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
