Fexapotide triflutate vs oral pharmacotherapy as initial therapy for moderate-to-severe benign prostate hyperplasia patients: a cost-effectiveness analysis
- PMID: 35550071
- PMCID: PMC9102263
- DOI: 10.1186/s12894-022-01025-4
Fexapotide triflutate vs oral pharmacotherapy as initial therapy for moderate-to-severe benign prostate hyperplasia patients: a cost-effectiveness analysis
Abstract
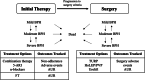
Background: To assess the price range in which fexapotide triflutate (FT), a novel injectable, is cost-effective relative to current oral pharmacotherapy (5 α-reductase inhibitor, α-blocker, 5 α-reductase inhibitor and α-blocker combination therapy) as initial therapy followed by surgery for moderate-to-severe benign prostate hyperplasia patients with lower urinary tract symptoms (BPH-LUTS).
Methods: We developed a microsimulation decision-analytic model to track the progression of BPH-LUTS and associated costs and quality-adjusted life years in the target population. The cost-effectiveness analysis was performed from Medicare's perspective with a time horizon of 4 years using 2019 US dollars for all costs. The microsimulation model considered treatment patterns associated with nonadherence to oral medication and progression to surgery. Model parameters were estimated from large randomized controlled trials, literature and expert opinion. For each initial treatment option, simulations were performed with 1000 iterations, with 1000 patients per iteration.
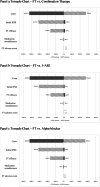
Results: Three upfront oral pharmacotherapy options are close in cost-effectiveness, with combination therapy being the most cost-effective option. Relative to upfront oral pharmacotherapy options, FT slightly increases quality-adjusted life years (QALY) per patient (1.870 (95% CI, 1.868 to 1.872) vs. 1.957 (95% CI, 1.955 to 1.959) QALYs). Under the willingness-to-pay (WTP) threshold of $150,000 per QALY, at price per injection of $14,000, FT is about as cost-effective as upfront oral pharmacotherapy options with net monetary benefit (NMB) $279,168.54. Under the WTP threshold of $50,000 per QALY, at price per injection of $5,000, FT is about as cost-effective as upfront oral pharmacotherapy options with NMB $92,135.18. In an alternative 10-year time horizon scenario, FT price per injection at $11,000 and $4,500 makes FT as cost-effective as oral pharmacotherapies. One-way sensitivity analysis showed this result is most sensitive to upfront therapy prices, FT efficacy and initial IPSS. At price per injections of $5,000, $10,000 and $15,000, the probability that FT is either cost-effective or dominant compared to upfront oral pharmacotherapy options using a WTP threshold of $150,000 per QALY is 100%, 93% and 40%, respectively.
Conclusions: Compared to upfront oral pharmacotherapy options, FT would be cost-effective at a price per injection below $14,000, assuming a WTP threshold of $150,000 per QALY.
Keywords: Benign prostate hyperplasia; Cost-effectiveness analysis; Decision analysis; Microsimulation model.
© 2022. The Author(s).
Conflict of interest statement
ARH reports personal fees from Nymox Pharmaceutical Corporation as paid consultant and being a shareholder in Nymox Pharmaceutical Corporation, outside the submitted work. YW, JWH and SS have nothing to disclose.
Figures

Comment in
-
Benign Prostatic Hyperplasia.J Urol. 2023 Feb;209(2):422-424. doi: 10.1097/JU.0000000000003044. Epub 2022 Nov 1. J Urol. 2023. PMID: 36318730 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

