Decreasing dorsal cochlear nucleus activity ameliorates noise-induced tinnitus perception in mice
- PMID: 35550106
- PMCID: PMC9097071
- DOI: 10.1186/s12915-022-01288-1
Decreasing dorsal cochlear nucleus activity ameliorates noise-induced tinnitus perception in mice
Abstract
Background: The dorsal cochlear nucleus (DCN) is a region known to integrate somatosensory and auditory inputs and is identified as a potential key structure in the generation of phantom sound perception, especially noise-induced tinnitus. Yet, how altered homeostatic plasticity of the DCN induces and maintains the sensation of tinnitus is not clear. Here, we chemogenetically decrease activity of a subgroup of DCN neurons, Ca2+/Calmodulin kinase 2 α (CaMKII α)-positive DCN neurons, using Gi-coupled human M4 Designer Receptors Exclusively Activated by Designer Drugs (hM4Di DREADDs), to investigate their role in noise-induced tinnitus.
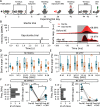
Results: Mice were exposed to loud noise (9-11kHz, 90dBSPL, 1h, followed by 2h of silence), and auditory brainstem responses (ABRs) and gap prepulse inhibition of acoustic startle (GPIAS) were recorded 2 days before and 2 weeks after noise exposure to identify animals with a significantly decreased inhibition of startle, indicating tinnitus but without permanent hearing loss. Neuronal activity of CaMKII α+ neurons expressing hM4Di in the DCN was lowered by administration of clozapine-N-oxide (CNO). We found that acutely decreasing firing rate of CaMKII α+ DCN units decrease tinnitus-like responses (p = 3e -3, n = 11 mice), compared to the control group that showed no improvement in GPIAS (control virus; CaMKII α-YFP + CNO, p = 0.696, n = 7 mice). Extracellular recordings confirmed CNO to decrease unit firing frequency of CaMKII α-hM4Di+ mice and alter best frequency and tuning width of response to sound. However, these effects were not seen if CNO had been previously administered during the noise exposure (n = 6 experimental and 6 control mice).
Conclusion: We found that lowering DCN activity in mice displaying tinnitus-related behavior reduces tinnitus, but lowering DCN activity during noise exposure does not prevent noise-induced tinnitus. Our results suggest that CaMKII α-positive cells in the DCN are not crucial for tinnitus induction but play a significant role in maintaining tinnitus perception in mice.
Keywords: Chemogenetics; Dorsal cochlear nucleus; GPIAS; Tinnitus; Unit recording.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
-
- Møller AR. Tinnitus: presence and future. Tinnitus Pathophysiol Treat. 2007;3–16. 10.1016/s0079-6123(07)66001-4.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

