NOTCH-YAP1/TEAD-DNMT1 Axis Drives Hepatocyte Reprogramming Into Intrahepatic Cholangiocarcinoma
- PMID: 35550144
- PMCID: PMC9329208
- DOI: 10.1053/j.gastro.2022.05.007
NOTCH-YAP1/TEAD-DNMT1 Axis Drives Hepatocyte Reprogramming Into Intrahepatic Cholangiocarcinoma
Abstract
Background & aims: Intrahepatic cholangiocarcinoma (ICC) is a devastating liver cancer with extremely high intra- and inter-tumoral molecular heterogeneity, partly due to its diverse cellular origins. We investigated clinical relevance and the molecular mechanisms underlying hepatocyte (HC)-driven ICC development.
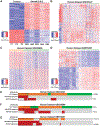
Methods: Expression of ICC driver genes in human diseased livers at risk for ICC development were examined. The sleeping beauty and hydrodynamic tail vein injection based Akt-NICD/YAP1 ICC model was used to investigate pathogenetic roles of SRY-box transcription factor 9 (SOX9) and yes-associated protein 1 (YAP1) in HC-driven ICC. We identified DNA methyltransferase 1 (DNMT1) as a YAP1 target, which was validated by loss- and gain-of-function studies, and its mechanism addressed by chromatin immunoprecipitation sequencing.
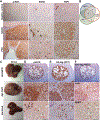
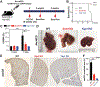
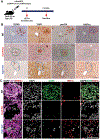
Results: Co-expression of AKT and Notch intracellular domain (NICD)/YAP1 in HC yielded ICC that represents 13% to 29% of clinical ICC. NICD independently regulates SOX9 and YAP1 and deletion of either, significantly delays ICC development. Yap1 or TEAD inhibition, but not Sox9 deletion, impairs HC-to-biliary epithelial cell (BEC) reprogramming. DNMT1 was discovered as a novel downstream effector of YAP1-TEAD complex that directs HC-to-BEC/ICC fate switch through the repression of HC-specific genes regulated by master regulators for HC differentiation, including hepatocyte nuclear factor 4 alpha, hepatocyte nuclear factor 1 alpha, and CCAAT/enhancer-binding protein alpha/beta. DNMT1 loss prevented NOTCH/YAP1-dependent HC-driven cholangiocarcinogenesis, and DNMT1 re-expression restored ICC development following TEAD repression. Co-expression of DNMT1 with AKT was sufficient to induce tumor development including ICC. DNMT1 was detected in a subset of HCs and dysplastic BECs in cholestatic human livers prone to ICC development.
Conclusion: We identified a novel NOTCH-YAP1/TEAD-DNMT1 axis essential for HC-to-BEC/ICC conversion, which may be relevant in cholestasis-to-ICC pathogenesis in the clinic.
Keywords: Bile Duct; Epigenetics; Liver Cancer; Precision Medicine; Transdifferentiation.
Copyright © 2022 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- National Cancer Institute NIoH. Cancer Stat Facts: Liver and Intrahepatic Bile Duct Cancer, 2018.
-
- Dabney RS, Khalife M, Shahid K, et al. Molecular pathways and targeted therapy in cholangiocarcinoma. Clin Adv Hematol Oncol 2019;17:630–637. - PubMed
-
- Lamarca A, Barriuso J, McNamara MG, et al. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J Hepatol 2020. - PubMed
-
- Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261–80. - PubMed
-
- Cai Y, Cheng N, Ye H, et al. The current management of cholangiocarcinoma: A comparison of current guidelines. Biosci Trends 2016;10:92–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK101426/DK/NIDDK NIH HHS/United States
- R01 DK116993/DK/NIDDK NIH HHS/United States
- R01 CA258449/CA/NCI NIH HHS/United States
- F30 DK121393/DK/NIDDK NIH HHS/United States
- P30 DK120531/DK/NIDDK NIH HHS/United States
- R01 DK124351/DK/NIDDK NIH HHS/United States
- R01 CA204586/CA/NCI NIH HHS/United States
- R01 DK130949/DK/NIDDK NIH HHS/United States
- R01 CA250227/CA/NCI NIH HHS/United States
- K01 DK110264/DK/NIDDK NIH HHS/United States
- R01 DK119435/DK/NIDDK NIH HHS/United States
- R01 CA251155/CA/NCI NIH HHS/United States
- T32 EB001026/EB/NIBIB NIH HHS/United States
- R01 CA124414/CA/NCI NIH HHS/United States
- R01 DK062277/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

