Beta-hydroxybutyrate dampens adipose progenitors' profibrotic activation through canonical Tgfβ signaling and non-canonical ZFP36-dependent mechanisms
- PMID: 35550189
- PMCID: PMC9123279
- DOI: 10.1016/j.molmet.2022.101512
Beta-hydroxybutyrate dampens adipose progenitors' profibrotic activation through canonical Tgfβ signaling and non-canonical ZFP36-dependent mechanisms
Abstract
Background/purpose: Adipose tissue contains progenitor cells that contribute to beneficial tissue expansion when needed by de novo adipocyte formation (classical white or beige fat cells with thermogenic potential). However, in chronic obesity, they can exhibit an activated pro-fibrotic, extracellular matrix (ECM)-depositing phenotype that highly aggravates obesity-related adipose tissue dysfunction.
Methods: Given that progenitors' fibrotic activation and fat cell browning appear to be antagonistic cell fates, we have examined the anti-fibrotic potential of pro-browning agents in an obesogenic condition.
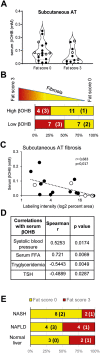
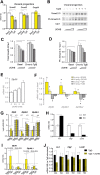
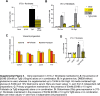
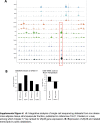
Results: In obese mice fed a high fat diet, thermoneutral housing, which induces brown fat cell dormancy, increases the expression of ECM gene programs compared to conventionally raised animals, indicating aggravation of obesity-related tissue fibrosis at thermoneutrality. In a model of primary cultured murine adipose progenitors, we found that exposure to β-hydroxybutyrate selectively reduced Tgfβ-dependent profibrotic responses of ECM genes like Ctgf, Loxl2 and Fn1. This effect is observed in both subcutaneous and visceral-derived adipose progenitors, as well as in 3T3-L1 fibroblasts. In 30 patients with obesity eligible for bariatric surgery, those with higher circulating β-hydroxybutyrate levels have lower subcutaneous adipose tissue fibrotic scores. Mechanistically, β-hydroxybutyrate limits Tgfβ-dependent collagen accumulation and reduces Smad2-3 protein expression and phosphorylation in visceral progenitors. Moreover, β-hydroxybutyrate induces the expression of the ZFP36 gene, encoding a post-transcriptional regulator that promotes the degradation of mRNA by binding to AU-rich sites within 3'UTRs. Importantly, complete ZFP36 deficiency in a mouse embryonic fibroblast line from null mice, or siRNA knock-down in primary progenitors, indicate that ZFP36 is required for β-hydroxybutyrate anti-fibrotic effects.
Conclusion: These data unravel the potential of β-hydroxybutyrate to limit adipose tissue matrix deposition, a finding that might exploited in an obesogenic context.
Keywords: Adipocyte; Extracellular matrix; Fibrosis; Progenitors.
Copyright © 2022 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annual Review of Physiology. 2010;72(1):219–246. - PubMed
-
- Marcelin G., Ferreira A., Liu Y., Atlan M., Aron-Wisnewsky J., Pelloux V., et al. A pdgfrα-mediated switch toward CD9highAdipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metabolism. 2017;25(3):673–685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

