The monoterpene 1,8-cineole prevents cerebral edema in a murine model of severe malaria
- PMID: 35550638
- PMCID: PMC9098050
- DOI: 10.1371/journal.pone.0268347
The monoterpene 1,8-cineole prevents cerebral edema in a murine model of severe malaria
Abstract
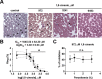
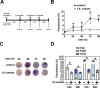
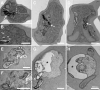
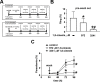
1,8-Cineole is a naturally occurring compound found in essential oils of different plants and has well-known anti-inflammatory and antimicrobial activities. In the present work, we aimed to investigate its potential antimalarial effect, using the following experimental models: (1) the erythrocytic cycle of Plasmodium falciparum; (2) an adhesion assay using brain microvascular endothelial cells; and (3) an experimental cerebral malaria animal model induced by Plasmodium berghei ANKA infection in susceptible mice. Using the erythrocytic cycle of Plasmodium falciparum, we characterized the schizonticidal effect of 1,8-cineole. This compound decreased parasitemia in a dose-dependent manner with a half maximal inhibitory concentration of 1045.53 ± 63.30 μM. The inhibitory effect of 972 μM 1,8-cineole was irreversible and independent of parasitemia. Moreover, 1,8-cineole reduced the progression of intracellular development of the parasite over 2 cycles, inducing important morphological changes. Ultrastructure analysis revealed a massive loss of integrity of endomembranes and hemozoin crystals in infected erythrocytes treated with 1,8-cineole. The monoterpene reduced the adhesion index of infected erythrocytes to brain microvascular endothelial cells by 60%. Using the experimental cerebral malaria model, treatment of infected mice for 6 consecutive days with 100 mg/kg/day 1,8-cineole reduced cerebral edema with a 50% reduction in parasitemia. Our data suggest a potential antimalarial effect of 1,8-cineole with an impact on the parasite erythrocytic cycle and severe disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- World Health Organization. World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

