E-Consent-a guide to maintain recruitment in clinical trials during the COVID-19 pandemic
- PMID: 35550639
- PMCID: PMC9096749
- DOI: 10.1186/s13063-022-06333-6
E-Consent-a guide to maintain recruitment in clinical trials during the COVID-19 pandemic
Abstract
Background: The COVID-19 pandemic has posed daunting challenges when conducting clinical research. Adopting new technologies such as remote electronic consent (e-Consent) can help overcome them. However, guidelines for e-Consent implementation in ongoing clinical trials are currently lacking. The NeuroSAFE PROOF trial is a randomized clinical trial evaluating the role of frozen section analysis during RARP for prostate cancer. In response to the COVID-19 crisis, recruitment was halted, and a remote e-Consent solution was designed. The aim of this paper is to describe the process of implementation, impact on recruitment rate, and patients' experience using e-Consent.
Methods: A substantial amendment of the protocol granted the creation of a remote e-Consent framework based on the REDCap environment, following the structure and content of the already approved paper consent form. Although e-Consent obviated the need for in-person meeting, there was nonetheless counselling sessions performed interactively online. This new pathway offered continuous support to patients through remote consultations. The whole process was judged to be compliant with regulatory requirements before implementation.
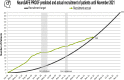
Results: Before the first recruitment suspension, NeuroSAFE PROOF was recruiting an average of 9 patients per month. After e-Consent implementation, 63 new patients (4/month) have been enrolled despite a second lockdown, none of whom would have been recruited using the old methods given restrictions on face-to-face consultations. Patients have given positive feedback on the use of the platform. Limited troubleshooting has been required after implementation.
Conclusion: Remote e-Consent-based recruitment was critical for the continuation of the NeuroSAFE PROOF trial during the COVID-19 pandemic. The described pathway complies with ethical and regulatory guidelines for informed consent, while minimizing face-to-face interactions that increase the risk of COVID-19 transmission. This guide will help researchers integrate e-Consent to ongoing or planned clinical trials while uncertainty about the course of the pandemic continues.
Trial registration: NeuroSAFE PROOF trial NCT03317990 . Registered on 23 October 2017. Regional Ethics Committee reference 17/LO/1978.
Keywords: Consent management; Informed consent; Prostate-cancer; e-Consent.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 3 Dec 2021.
-
- NHS Health Research Authority. Making changes to a research study to manage the impact of COVID-19. https://www.hra.nhs.uk/covid-19-research/covid-19-guidance-sponsors-site... Accessed 3 Decer 2021.
-
- FDA. Conduct of clinical trials of medical products during the COVID-19 public health emergency 2020. https://www.fda.gov/media/136238/download. Accessed 3 Dec 2021.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical