Efficacy of niraparib by time of surgery and postoperative residual disease status: A post hoc analysis of patients in the PRIMA/ENGOT-OV26/GOG-3012 study
- PMID: 35550709
- PMCID: PMC10025898
- DOI: 10.1016/j.ygyno.2022.04.012
Efficacy of niraparib by time of surgery and postoperative residual disease status: A post hoc analysis of patients in the PRIMA/ENGOT-OV26/GOG-3012 study
Abstract
Objective: To evaluate the association between surgical timing and postoperative residual disease status on the efficacy of niraparib first-line maintenance therapy in patients with newly diagnosed advanced ovarian cancer at high risk of recurrence.
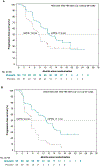
Methods: Post hoc analysis of the phase 3 PRIMA/ENGOT-OV26/GOG-3012 (NCT02655016) study of niraparib in patients with newly diagnosed primary advanced ovarian, primary peritoneal, or fallopian tube cancer with a complete/partial response to first-line platinum-based chemotherapy. Progression-free survival (PFS) was assessed by surgical status (primary debulking surgery [PDS] vs neoadjuvant chemotherapy/interval debulking surgery [NACT/IDS]) and postoperative residual disease status (no visible residual disease [NVRD] vs visible residual disease [VRD]) in the intent-to-treat population.
Results: In PRIMA (N = 733), 236 (32.2%) patients underwent PDS, and 481 (65.6%) received NACT/IDS before enrollment. Median PFS (niraparib vs placebo) and hazard ratios (95% CI) for progression were similar in PDS (13.7 vs 8.2 months; HR, 0.67 [0.47-0.96]) and NACT/IDS (14.2 vs 8.2 months; HR, 0.57 [0.44-0.73]) subgroups. In patients who received NACT/IDS and had NVRD (n = 304), the hazard ratio (95% CI) for progression was 0.65 (0.46-0.91). In patients with VRD following PDS (n = 183) or NACT/IDS (n = 149), the hazard ratios (95% CI) for progression were 0.58 (0.39-0.86) and 0.41 (0.27-0.62), respectively. PFS was not evaluable for patients with PDS and NVRD because of sample size (n = 37).
Conclusions: In this post hoc analysis, niraparib efficacy was similar across PDS and NACT/IDS subgroups. Patients who had NACT/IDS and VRD had the highest reduction in the risk of progression with niraparib maintenance.
Keywords: Maintenance therapy; Niraparib; Ovarian cancer; PARP inhibitor; Surgery.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest REO reports participating in advisory boards with Bayer, Fresenius Kabi, GlaxoSmithKline, Immunogen, Regeneron, and Seattle Genetics; personal fees from GOG Foundation; and service as a noncompensated steering committee member for the PRIMA (niraparib) and DUO-O (olaparib) studies; institutional research support grants from AstraZeneca/Merck, Atara Biotherapeutics/Bayer, Genentech, Genmab, GlaxoSmithKline, Gynecologic Oncology Group Foundation, Juno Therapeutics, Kite/Gilead, Ludwig Institute for Cancer Research, Regeneron, Sellas Life Sciences, Stem CentRx, Syndax, TapImmune Inc., and TCR2 Therapeutics. JAPF reports personal fees from Abilify Pharma, Amgen, AstraZeneca, Clinigen, Clovis, GlaxoSmithKline, and Roche; and institutional grants from GlaxoSmithKline. BJM reports consulting fees from Agenus, Akeso Bio, Amgen, Aravive, Bayer, Elevar, EMD Merck, Genmab/Seagen, GOG Foundation, Gradalis, ImmunoGen, Iovance, Karyopharm, Macrogenics, Mersana, Myriad, Novartis, Novocure, Regeneron, Sorrento, Pfizer, Puma, US Oncology Research, and VBL; speaker's bureau honoraria from AstraZeneca, Clovis, Eisai, Merck, Roche/Genentech, TESARO/GSK; and is an investigator for US Oncology Research. IT reports personal fees from Celgene and Roche Pharma AG; institutional grants from Roche; and travel support from Roche. RGM reports personal fees from Abcodia Inc., Fujirebio Diagnostics Inc., and Humphries Pharmaceutical; and institutional grants from Angle Plc. MSS reports personal fees from AstraZeneca, Clovis Oncology, GlaxoSmithKline, Merck, and Pacira Pharmaceuticals Inc.; and institutional grants from GlaxoSmithKline. DO reports personal fees from Agenus, Eisai, GlaxoSmithKline, and Immunogen; consultant/advisory board for Abbvie, Ambry, Amgen, Array Biopharma, Clovis, EMD Serono, Ergomed, Janssen/J&J, INC Research Inc., inVentiv Health Clinical, Iovance Biotherapeutics Inc., Myriad Genetics, Novacure, Regeneron, Tarveda, and VentiRx; steering committee for Genentech/Roche and Merck; institutional funding from Ajinomoto Inc., Bristol Myers Squibb, Cerulean Pharma, GOG Foundation, Ludwig Cancer Research, New Mexico Cancer Care Alliance, PRA International, Stemcentrx Inc., Serono Inc., Tracon Pharmaceuticals, and Yale University. BMS reports consulting/advisory fees from Abbvie, AstraZeneca, Clovis, Eisai, Genentech, GlaxoSmithKline, GOG Foundation, Merck, and Myriad. FS reports personal fees from AstraZeneca, Clovis, GlaxoSmithKline, MSD, PharmaMar, and Roche; and travel support from AstraZeneca, GlaxoSmithKline, MSD, PharmaMar, and Roche. AK reports personal fees from AstraZeneca and GlaxoSmithKline. AGM reports consulting or advisory roles at Amgen, AstraZeneca, Clovis, Genmab, GlaxoSmithKline, Merck & Co., Mersana, Immunogen, Roche, Sotio, and Takeda; speaker's bureau compensation from AstraZeneca, Clovis, GlaxoSmithKline, Merck & Co., and Roche; institutional research funding from Roche and GlaxoSmithKline; and travel support from AstraZeneca, GlaxoSmithKline, and Roche. CM, CV, JF, FF, WHB, SH, AD, KB, PMC, GA, and TL have nothing to disclose. IAM and DG are employees of GlaxoSmithKline. YL was an employee at GlaxoSmithKline at the time of the analysis; currently an employee of Adagio Therapeutics Inc.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. , Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J. Clin 71 (3) (2021) 209–249. - PubMed
-
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. , SEER cancer Statistics Review, National Cancer Institute, Bethesda, MD, 2020.
-
- Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. , Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up, Ann. Oncol 24 Suppl 6 (2013) vi24–32. - PubMed
-
- Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. , Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline, J. Clin. Oncol 34 (28) (2016) 3460–3473. - PMC - PubMed

