Immunoglobulin repletion during blinatumomab therapy does not reduce the rate of secondary hypogammaglobulinemia and associated infectious risk
- PMID: 35551109
- PMCID: PMC9242831
- DOI: 10.5045/br.2022.2021163
Immunoglobulin repletion during blinatumomab therapy does not reduce the rate of secondary hypogammaglobulinemia and associated infectious risk
Abstract
Background: Blinatumomab has demonstrated efficacy in minimal residual disease (MRD) positive and relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) by inciting rapid and sustained B-cell depletion.
Methods: Owing to its effect on B-cells, blinatumomab is associated with a higher rate of secondary hypogammaglobulinemia compared to chemotherapy. To mitigate blinatumomab-induced hypogammaglobulinemia, patients were pre-emptively repleted with intravenous immune globulin (IVIG) during blinatumomab therapy. In this retrospective study, we compared outcomes of 23 blinatumomab treated adults with ALL. Seventeen patients routinely received IVIG and 6 patients were in the control cohort.
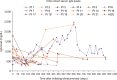
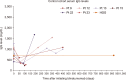
Results: Our findings demonstrated no difference between the two cohorts in immunoglobulin G (IgG) nadir (338 mg/dL vs. 337 mg/dL, P=0.641), days to IgG nadir (120.5 vs. 85.5 days, P=0.13), infection rate (82.4% vs. 66.7%, P=0.58), infections requiring ICU admission (23.5% vs. 16.7%, P=1), and infection related mortality (17.6% vs. 16.7%, P=1).
Conclusion: Pre-emptive IVIG repletion during blinatumomab did not prevent hypogammaglobulinemia and associated infection risk.
Keywords: ALL; Acute lymphoblastic leukemia; Blinatumomab; Hypogammaglobulinemia; Intravenous Immunoglobulin.
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
Figures
Similar articles
-
Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children With High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial.JAMA. 2021 Mar 2;325(9):843-854. doi: 10.1001/jama.2021.0987. JAMA. 2021. PMID: 33651091 Free PMC article. Clinical Trial.
-
Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival.J Clin Oncol. 2011 Jun 20;29(18):2493-8. doi: 10.1200/JCO.2010.32.7270. Epub 2011 May 16. J Clin Oncol. 2011. PMID: 21576633 Clinical Trial.
-
Extramedullary disease relapse and progression after blinatumomab therapy for treatment of acute lymphoblastic leukemia.Cancer. 2022 Feb 1;128(3):529-535. doi: 10.1002/cncr.33967. Epub 2021 Oct 11. Cancer. 2022. PMID: 34633671
-
Evaluating Blinatumomab for the Treatment of Relapsed/Refractory ALL: Design, Development, and Place in Therapy.Blood Lymphat Cancer. 2020 Nov 3;10:7-20. doi: 10.2147/BLCTT.S223894. eCollection 2020. Blood Lymphat Cancer. 2020. PMID: 33173373 Free PMC article. Review.
-
Impact of blinatumomab on patient outcomes in relapsed/refractory acute lymphoblastic leukemia: evidence to date.Patient Relat Outcome Meas. 2018 Oct 2;9:329-337. doi: 10.2147/PROM.S149420. eCollection 2018. Patient Relat Outcome Meas. 2018. PMID: 30323696 Free PMC article. Review.
Cited by
-
Case Report: Blinatumomab as upfront consolidation and maintenance therapy in a pediatric patient with high-risk B-cell acute lymphoblastic leukemia.Front Oncol. 2023 Nov 1;13:1246924. doi: 10.3389/fonc.2023.1246924. eCollection 2023. Front Oncol. 2023. PMID: 38023197 Free PMC article.
-
Blinatumomab in Children with MRD-Positive B-Cell Precursor Acute Lymphoblastic Leukemia: A Report of 11 Cases.Hematol Rep. 2024 Jun 3;16(2):347-353. doi: 10.3390/hematolrep16020035. Hematol Rep. 2024. PMID: 38921183 Free PMC article.
-
Blinatumomab in Practice.Curr Hematol Malig Rep. 2024 Feb;19(1):1-8. doi: 10.1007/s11899-023-00714-7. Epub 2023 Dec 7. Curr Hematol Malig Rep. 2024. PMID: 38060085 Review.
References
-
- National Comprehensive Cancer Network, author. Acute lymphoblastic leukemia (version 2.2020) National Comprehensive Cancer Network; Plymouth Meeting, PA: 2020. [Accessed November 28, 2020]. at https://www.nccn.org/professionals/physician_gls/pdf/all.pdf .
LinkOut - more resources
Full Text Sources