Dissociable multi-scale patterns of development in personalized brain networks
- PMID: 35551181
- PMCID: PMC9098559
- DOI: 10.1038/s41467-022-30244-4
Dissociable multi-scale patterns of development in personalized brain networks
Abstract
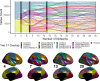
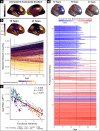
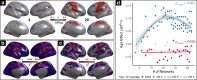
The brain is organized into networks at multiple resolutions, or scales, yet studies of functional network development typically focus on a single scale. Here, we derive personalized functional networks across 29 scales in a large sample of youths (n = 693, ages 8-23 years) to identify multi-scale patterns of network re-organization related to neurocognitive development. We found that developmental shifts in inter-network coupling reflect and strengthen a functional hierarchy of cortical organization. Furthermore, we observed that scale-dependent effects were present in lower-order, unimodal networks, but not higher-order, transmodal networks. Finally, we found that network maturation had clear behavioral relevance: the development of coupling in unimodal and transmodal networks are dissociably related to the emergence of executive function. These results suggest that the development of functional brain networks align with and refine a hierarchy linked to cognition.
© 2022. The Author(s).
Conflict of interest statement
R.T.S. has consulting income from Genentech/Roche and Octave Bioscience. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

