Stress-induced changes of the cholinergic circuitry promote retrieval-based generalization of aversive memories
- PMID: 35551246
- PMCID: PMC9846583
- DOI: 10.1038/s41380-022-01610-x
Stress-induced changes of the cholinergic circuitry promote retrieval-based generalization of aversive memories
Erratum in
-
Correction: Stress-induced changes of the cholinergic circuitry promote retrieval-based generalization of aversive memories.Mol Psychiatry. 2022 Sep;27(9):3806. doi: 10.1038/s41380-022-01650-3. Mol Psychiatry. 2022. PMID: 35681082 Free PMC article. No abstract available.
Abstract
Generalization, the process of applying knowledge acquired in one context to other contexts, often drives the expression of similar behaviors in related situations. At the cellular level, generalization is thought to depend on the activity of overlapping neurons that represent shared features between contexts (general representations). Using contextual fear conditioning in mice, we demonstrate that generalization can also occur in response to stress and result from reactivation of specific, rather than general context representations. We found that generalization emerges during memory retrieval, along with stress-induced abnormalities of septohippocampal oscillatory activity and acetylcholine release, which are typically found in negative affective states. In hippocampal neurons that represent aversive memories and drive generalization, cholinergic septohippocampal afferents contributed to a unique reactivation pattern of cFos, Npas4, and repressor element-1 silencing transcription factor (REST). Together, these findings suggest that generalization can be triggered by perceptually dissimilar but valence-congruent memories of specific aversive experiences. Through promoting the reactivation of such memories and their interference with ongoing behavior, abnormal cholinergic signaling could underlie maladaptive cognitive and behavioral generalization linked to negative affective states.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing financial interests.
Figures
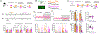

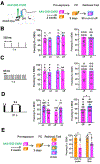


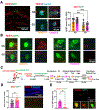
Similar articles
-
Activity of the anterior cingulate cortex and ventral hippocampus underlie increases in contextual fear generalization.Neurobiol Learn Mem. 2015 Oct;124:19-27. doi: 10.1016/j.nlm.2015.07.001. Epub 2015 Jul 9. Neurobiol Learn Mem. 2015. PMID: 26165137
-
Hippocampal Engrams and Contextual Memory.Adv Neurobiol. 2024;38:45-66. doi: 10.1007/978-3-031-62983-9_4. Adv Neurobiol. 2024. PMID: 39008010 Free PMC article. Review.
-
Artificially Enhancing and Suppressing Hippocampus-Mediated Memories.Curr Biol. 2019 Jun 3;29(11):1885-1894.e4. doi: 10.1016/j.cub.2019.04.065. Epub 2019 May 23. Curr Biol. 2019. PMID: 31130452 Free PMC article.
-
Nucleus Reuniens Is Required for Encoding and Retrieving Precise, Hippocampal-Dependent Contextual Fear Memories in Rats.J Neurosci. 2018 Nov 14;38(46):9925-9933. doi: 10.1523/JNEUROSCI.1429-18.2018. Epub 2018 Oct 3. J Neurosci. 2018. PMID: 30282726 Free PMC article.
-
Modulation of Aversive Memory by Adult Hippocampal Neurogenesis.Neurotherapeutics. 2017 Jul;14(3):646-661. doi: 10.1007/s13311-017-0528-9. Neurotherapeutics. 2017. PMID: 28488160 Free PMC article. Review.
Cited by
-
Dominant activities of fear engram cells in the dorsal dentate gyrus underlie fear generalization in mice.PLoS Biol. 2024 Jul 12;22(7):e3002679. doi: 10.1371/journal.pbio.3002679. eCollection 2024 Jul. PLoS Biol. 2024. PMID: 38995985 Free PMC article.
-
Septohippocampal cholinergic system at the intersection of stress and cognition: Current trends and translational implications.Eur J Neurosci. 2024 May;59(9):2155-2180. doi: 10.1111/ejn.15999. Epub 2023 May 10. Eur J Neurosci. 2024. PMID: 37118907 Free PMC article. Review.
-
Formation of memory assemblies through the DNA-sensing TLR9 pathway.Nature. 2024 Apr;628(8006):145-153. doi: 10.1038/s41586-024-07220-7. Epub 2024 Mar 27. Nature. 2024. PMID: 38538785 Free PMC article.
-
Distinct subpopulations of ventral pallidal cholinergic projection neurons encode valence of olfactory stimuli.bioRxiv [Preprint]. 2023 Oct 10:2023.10.06.561261. doi: 10.1101/2023.10.06.561261. bioRxiv. 2023. Update in: Cell Rep. 2024 Apr 23;43(4):114009. doi: 10.1016/j.celrep.2024.114009. PMID: 37986753 Free PMC article. Updated. Preprint.
-
Distinct subpopulations of ventral pallidal cholinergic projection neurons encode valence of olfactory stimuli.Cell Rep. 2024 Apr 23;43(4):114009. doi: 10.1016/j.celrep.2024.114009. Epub 2024 Mar 26. Cell Rep. 2024. PMID: 38536818 Free PMC article.
References
-
- Banich MT, Dukes P, & Caccamise D Generalization of knowledge: Multidisciplinary perspectives: Psychology Press; 2010.
-
- Ono M, Devilly GJ, Shum DH. A meta-analytic review of overgeneral memory: The role of trauma history, mood, and the presence of posttraumatic stress disorder. Psychol Trauma 2016;8(2):157–64. - PubMed
-
- Barry TJ, Chiu CPY, Raes F, Ricarte J, Lau H. The Neurobiology of Reduced Autobiographical Memory Specificity. Trends Cogn Sci 2018;22(11):1038–49. - PubMed
-
- King MJ, MacDougall AG, Ferris SM, Levine B, MacQueen GM, McKinnon MC. A review of factors that moderate autobiographical memory performance in patients with major depressive disorder. J Clin Exp Neuropsychol 2010;32(10):1122–44. - PubMed
-
- Bennett M, Vervoort E, Boddez Y, Hermans D, Baeyens F. Perceptual and conceptual similarities facilitate the generalization of instructed fear. J Behav Ther Exp Psychiatry 2015;48:149–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

