Chemical editing of proteoglycan architecture
- PMID: 35551261
- PMCID: PMC9205196
- DOI: 10.1038/s41589-022-01023-5
Chemical editing of proteoglycan architecture
Abstract
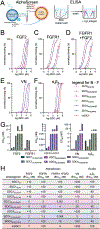
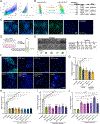
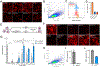
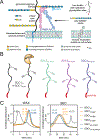
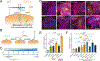
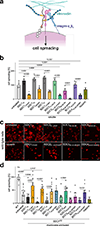
Proteoglycans are heterogeneous macromolecular glycoconjugates that orchestrate many important cellular processes. While much attention has focused on the poly-sulfated glycosaminoglycan chains that decorate proteoglycans, other important elements of their architecture, such as core proteins and membrane localization, have garnered less emphasis. Hence, comprehensive structure-function relationships that consider the replete proteoglycan architecture as glycoconjugates are limited. Here we present an extensive approach to study proteoglycan structure and biology by fabricating defined semisynthetic modular proteoglycans that can be tailored for cell surface display. The expression of proteoglycan core proteins with unnatural amino acids permits bioorthogonal click chemistry with functionalized glycosaminoglycans for methodical dissection of the parameters required for optimal binding and function of various proteoglycan-binding proteins. We demonstrate that these sophisticated materials can recapitulate the functions of native proteoglycan ectodomains in mouse embryonic stem cell differentiation and cancer cell spreading while permitting the analysis of the contributing architectural elements toward function.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests Statement
The authors declare no competing interests.
Figures






References
-
- Zhang YH et al. Targeting of Heparanase-modified Syndecan-1 by Prosecretory Mitogen Lacritin Requires Conserved Core GAGAL plus Heparan and Chondroitin Sulfate as a Novel Hybrid Binding Site That Enhances Selectivity. Journal of Biological Chemistry 288, 12090–12101, doi: 10.1074/jbc.M112.422717 (2013). - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

