Exogenous gonadotropin-releasing hormone counteracts the adverse effect of scrotal insulation on testicular functions in bucks
- PMID: 35551262
- PMCID: PMC9098548
- DOI: 10.1038/s41598-022-11884-4
Exogenous gonadotropin-releasing hormone counteracts the adverse effect of scrotal insulation on testicular functions in bucks
Abstract
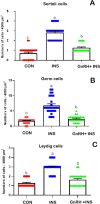
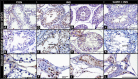
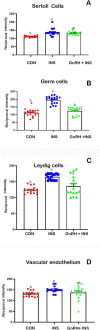
This study determined the effects of scrotal insulation on testicular functions in bucks and evaluated the impact of exogenous gonadotropin-releasing hormone (GnRH) administration before scrotal insulation on sperm production and testicular vascular dynamics. Twelve bucks were randomly divided into three groups: scrotal-insulated animals without GnRH treatment (INS), scrotal-insulated animals treated previously with GnRH (GnRH + INS), and animals without insulation as controls (CON). Doppler ultrasonography was used to evaluate testicular vascular changes, and semen samples were collected to assess seminal parameters. Testicular samples were collected from slaughtered bucks at the end of the experiment for histological investigations and immunohistochemical analysis for caspase 3 (apoptotic marker), and a vascular endothelial growth factor (VEGF; hypoxic marker) evaluation. Sperm motility drastically decreased (33%) in the INS group on day 8 compared with those in the GnRH + INS and CON groups (58% and 85%, respectively). Testicular blood flow significantly decreased for 3 and 2 weeks in the INS and GnRH + INS groups, respectively. The pulsatility index (PI) reached pretreatment values at 5 and 4 weeks after insulation in the INS and GnRH + INS groups, respectively. The resistance index (RI) values increased in both insulated groups for the first 2 weeks and decreased to control values 4 weeks after insulation. However, the maximum velocity (VP) started to increase reaching pretreatment values by the 5th and 3rd weeks after insulation in the INS and GnRH + INS groups, respectively. Histological investigations showed a marked reduction in lipid inclusions in Sertoli cells in the GnRH + INS group compared with those in the INS group. The distributions of both caspase 3 and VEGF decreased in the GnRH + INS group compared with those in the INS group. This study showed that the administration of a single dose of GnRH delayed the negative effects of scrotal insulation on different seminal traits and revealed the pivotal role of GnRH in compensating testicular insulation in bucks.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Correlation of testicular ultrasonography, testicular biometry, serum testosterone levels and seminal attributes in pre and post-pubertal age for breeding soundness evaluation in Osmanabadi bucks.Trop Anim Health Prod. 2019 Jul;51(6):1467-1480. doi: 10.1007/s11250-019-01834-0. Epub 2019 Feb 9. Trop Anim Health Prod. 2019. PMID: 30739277
-
Chronic use of a GnRH agonist (deslorelin) or immunization against GnRH: effects on testicular function and sperm quality of bucks.Domest Anim Endocrinol. 2020 Apr;71:106395. doi: 10.1016/j.domaniend.2019.106395. Epub 2019 Sep 14. Domest Anim Endocrinol. 2020. PMID: 31731252
-
Effects of scrotal insulation on sperm production, semen quality, and testicular echotexture in Bos indicus and Bos indicus x Bos taurus bulls.Anim Reprod Sci. 2003 Nov 20;79(1-2):1-15. doi: 10.1016/s0378-4320(03)00082-4. Anim Reprod Sci. 2003. PMID: 12853175
-
Review: Testicular vascular cone development and its association with scrotal thermoregulation, semen quality and sperm production in bulls.Animal. 2018 Jun;12(s1):s133-s141. doi: 10.1017/S1751731118001167. Animal. 2018. PMID: 29882506 Review.
-
RFamide-related peptide 3 and gonadotropin-releasing hormone-II are autocrine-paracrine regulators of testicular function in the boar.Mol Reprod Dev. 2017 Sep;84(9):994-1003. doi: 10.1002/mrd.22830. Epub 2017 Jun 21. Mol Reprod Dev. 2017. PMID: 28475264 Review.
Cited by
-
Microstructural architecture of the bony scutes, spine, and rays of the bony fins in the common pleco (Hypostomus plecostomus).Int J Vet Sci Med. 2024 Sep 4;12(1):101-124. doi: 10.1080/23144599.2024.2374201. eCollection 2024. Int J Vet Sci Med. 2024. PMID: 39239634 Free PMC article.
-
Systematic review of hormonal strategies to improve fertility in rams.Anim Reprod. 2024 Jun 10;21(2):e20240007. doi: 10.1590/1984-3143-AR2024-0007. eCollection 2024. Anim Reprod. 2024. PMID: 38903866 Free PMC article. Review.
-
The selection of Y chromosome microdeletion detection methods based on seminal analysis results: a comparison of high-throughput sequencing and fluorescence quantitative polymerase chain reaction (qPCR) applications.Transl Androl Urol. 2025 Mar 30;14(3):619-626. doi: 10.21037/tau-24-593. Epub 2025 Mar 26. Transl Androl Urol. 2025. PMID: 40226073 Free PMC article.
-
The synergistic impact of Spirulina and selenium nanoparticles mitigates the adverse effects of heat stress on the physiology of rabbits bucks.PLoS One. 2023 Jul 12;18(7):e0287644. doi: 10.1371/journal.pone.0287644. eCollection 2023. PLoS One. 2023. PMID: 37437098 Free PMC article.
References
-
- Santos DO, Simplício AA. Scrotal testicular and semen parameters in adult male goats submitted to scrotal insulation. Pesq. Agrop. Brasileira. 2000;35:1835–1841. doi: 10.1590/S0100-204X2000000900016. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

