Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial
- PMID: 35551292
- PMCID: PMC9117147
- DOI: 10.1038/s41591-022-01803-5
Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial
Abstract
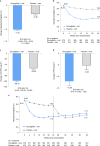
Metformin, the first-line therapy for type 2 diabetes (T2D), decreases hepatic glucose production and reduces fasting plasma glucose levels. Dorzagliatin, a dual-acting orally bioavailable glucokinase activator targeting both the pancreas and liver glucokinase, decreases postprandial glucose in patients with T2D. In this randomized, double-blind, placebo-controlled phase 3 trial, the efficacy and safety of dorzagliatin as an add-on therapy to metformin were assessed in patients with T2D who had inadequate glycemic control using metformin alone. Eligible patients with T2D (n = 767) were randomly assigned to receive dorzagliatin or placebo (1:1 ratio) as an add-on to metformin (1,500 mg per day) for 24 weeks of double-blind treatment, followed by 28 weeks of open-label treatment with dorzagliatin for all patients. The primary efficacy endpoint was the change in glycated hemoglobin (HbA1c) levels from baseline to week 24, and safety was assessed throughout the trial. At week 24, the least-squares mean change from baseline in HbA1c (95% confidence interval (CI)) was -1.02% (-1.11, -0.93) in the dorzagliatin group and -0.36% (-0.45, -0.26) in the placebo group (estimated treatment difference, -0.66%; 95% CI: -0.79, -0.53; P < 0.0001). The incidence of adverse events was similar between groups. There were no severe hypoglycemia events or drug-related serious adverse events in the dorzagliatin and metformin combined therapy group. In patients with T2D who experienced inadequate glycemic control with metformin alone, dorzagliatin resulted in effective glycemic control with good tolerability and safety profile ( NCT03141073 ).
© 2022. The Author(s).
Conflict of interest statement
L.Chen, Y.Zhao and Y.Zhang are employees of Hua Medicine. W.Yang, D.Zhu and X.Li have served on the Diabetes Advisory Board for Hua Medicine. The remaining authors declare no competing interests.
Figures

Comment in
-
A new class of drug in the diabetes toolbox.Nat Med. 2022 May;28(5):901-902. doi: 10.1038/s41591-022-01783-6. Nat Med. 2022. PMID: 35551293 No abstract available.
References
-
- International Diabetes Federation. IDF Diabetes Atlas 10th edn (International Diabetes Federation, 2021).
-
- American Diabetes Association Professional Practice Committee et al. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care45, S125–S143 (2022).. - PubMed
-
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK prospective diabetes study (UKPDS) group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

