Development and psychometric properties of the human papillomavirus-quality of life (HPV-QoL) questionnaire to assess the impact of HPV on women health-related-quality-of-life
- PMID: 35551456
- PMCID: PMC9470626
- DOI: 10.1007/s00404-022-06583-4
Development and psychometric properties of the human papillomavirus-quality of life (HPV-QoL) questionnaire to assess the impact of HPV on women health-related-quality-of-life
Abstract
Purpose: The HPV-Quality-of-Life (HPV-QoL) questionnaire was developed to determine the impact of Human-Papillomavirus (HPV) infection and related interventions on women health-related quality-of-life. This study provides the development and preliminary psychometric properties of a novel HPV-QoL questionnaire for adult women with HPV.
Methods: After reviewing literature and cognitive debriefing interviews in women who had experienced HPV-related conditions, instrument items and domains were developed. A draft questionnaire was pilot tested for comprehension and ease of completion. Psychometric evaluation of the final HPV-QoL scale was conducted in a psychometric study including 252 adult women derived to our centre by a positive HPV test in the cervical cancer screening program and/or presenting genital warts.
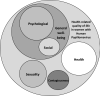
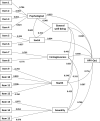
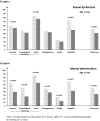
Results: The present study reveals that the HPV-QoL questionnaire, structured in four domains: general well-being [including psychological well-being and social well-being subdomains], health, contagiousness and sexuality, showed good metric properties of feasibility irrespective of age or educational level, and time to administer was less than 5 min. Internal consistency and temporal stability (reliability) showed values above the acceptable standards. The instrument showed its concurrent validity by means of a significant correlation with mental and sexual existing instruments; GHQ-12 and FSFI questionnaires, respectively, and also known groups validity showing significant differences among the subgroups regarding either sexual dysfunction or mental deterioration.
Conclusion: This study provides an HPV-QoL questionnaire with an innovative patient-reported outcomes specific measurement tool to assess HRQoL in women with HPV infection. The present study suggests this questionnaire has satisfactory psychometric properties, including validity and reliability. Results support the use of the HPV-QoL questionnaire as a HRQoL measurement instrument for daily medical practice and clinical research.
Keywords: Development; HPV-QoL questionnaire; Human papillomavirus (HPV) infection; Women health-related quality-of-life.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Population-based norms for the human papillomavirus-quality of life (HPV-QoL) questionnaire: A cross-sectional multicenter study.Acta Obstet Gynecol Scand. 2024 Aug;103(8):1584-1595. doi: 10.1111/aogs.14877. Epub 2024 Jun 13. Acta Obstet Gynecol Scand. 2024. PMID: 38872262 Free PMC article.
-
Development and psychometric properties of the HPV Impact Profile (HIP) to assess the psychosocial burden of HPV.Curr Med Res Opin. 2009 Nov;25(11):2609-19. doi: 10.1185/03007990903238786. Curr Med Res Opin. 2009. PMID: 19739938
-
Development and psychometric properties of a measurement to ascertain the impact of genitourinary symptoms on health-related quality of life in menopausal women: the Cervantes-GSM questionnaire.Menopause. 2023 May 1;30(5):512-520. doi: 10.1097/GME.0000000000002171. Epub 2023 Mar 12. Menopause. 2023. PMID: 36917753 Review.
-
Validation of the Human Papillomavirus Impact Profile in Lebanese Women With Human Papillomavirus or Associated Lesions.J Low Genit Tract Dis. 2022 Jan 1;26(1):2-7. doi: 10.1097/LGT.0000000000000640. J Low Genit Tract Dis. 2022. PMID: 34928247
-
[Psychometric characteristics of questionnaires designed to assess the knowledge, perceptions and practices of health care professionals with regards to alcoholic patients].Encephale. 2004 Sep-Oct;30(5):437-46. doi: 10.1016/s0013-7006(04)95458-9. Encephale. 2004. PMID: 15627048 Review. French.
Cited by
-
Population-based norms for the human papillomavirus-quality of life (HPV-QoL) questionnaire: A cross-sectional multicenter study.Acta Obstet Gynecol Scand. 2024 Aug;103(8):1584-1595. doi: 10.1111/aogs.14877. Epub 2024 Jun 13. Acta Obstet Gynecol Scand. 2024. PMID: 38872262 Free PMC article.
-
Sexual Function and Quality of Life in Iranian Women With Human Papillomavirus Infection.J Family Reprod Health. 2024 Sep;18(3):154-159. doi: 10.18502/jfrh.v18i3.16656. J Family Reprod Health. 2024. PMID: 39439738 Free PMC article.
-
Translation and psychometrics of the HPV impact profile in Iranian women.BMC Womens Health. 2025 Apr 1;25(1):151. doi: 10.1186/s12905-025-03678-3. BMC Womens Health. 2025. PMID: 40169973 Free PMC article.
-
Sexual dysfunction in women with genital warts: a cross-sectional study.BMC Womens Health. 2025 Apr 16;25(1):188. doi: 10.1186/s12905-025-03691-6. BMC Womens Health. 2025. PMID: 40241142 Free PMC article.

