Aniridia-related keratopathy relevant cell signaling pathways in human fetal corneas
- PMID: 35551459
- PMCID: PMC9338123
- DOI: 10.1007/s00418-022-02099-9
Aniridia-related keratopathy relevant cell signaling pathways in human fetal corneas
Abstract
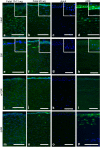
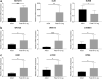
We aimed to study aniridia-related keratopathy (ARK) relevant cell signaling pathways [Notch1, Wnt/β-catenin, Sonic hedgehog (SHH) and mTOR] in normal human fetal corneas compared with normal human adult corneas and ARK corneas. We found that fetal corneas at 20 weeks of gestation (wg) and normal adult corneas showed similar staining patterns for Notch1; however 10-11 wg fetal corneas showed increased presence of Notch1. Numb and Dlk1 had an enhanced presence in the fetal corneas compared with the adult corneas. Fetal corneas showed stronger immunolabeling with antibodies against β-catenin, Wnt5a, Wnt7a, Gli1, Hes1, p-rpS6, and mTOR when compared with the adult corneas. Gene expression of Notch1, Wnt5A, Wnt7A, β-catenin, Hes1, mTOR, and rps6 was higher in the 9-12 wg fetal corneas compared with adult corneas. The cell signaling pathway differences found between human fetal and adult corneas were similar to those previously found in ARK corneas with the exception of Notch1. Analogous profiles of cell signaling pathway activation between human fetal corneas and ARK corneas suggests that there is a less differentiated host milieu in ARK.
Keywords: Adult cornea; Aniridia; Fetal cornea; Notch; Sonic hedgehog; Wnt; mTOR.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


Similar articles
-
Altered Signaling Pathways in Aniridia-Related Keratopathy.Invest Ophthalmol Vis Sci. 2018 Nov 1;59(13):5531-5541. doi: 10.1167/iovs.18-25175. Invest Ophthalmol Vis Sci. 2018. PMID: 30480741
-
Aniridia-related keratopathy: Structural changes in naïve and transplanted corneal buttons.PLoS One. 2018 Jun 11;13(6):e0198822. doi: 10.1371/journal.pone.0198822. eCollection 2018. PLoS One. 2018. PMID: 29889891 Free PMC article.
-
Sonic Hedgehog pathway is upregulated in adamantinomatous craniopharyngiomas.Eur J Endocrinol. 2015 May;172(5):603-8. doi: 10.1530/EJE-14-0934. Epub 2015 Feb 18. Eur J Endocrinol. 2015. PMID: 25693592
-
Effects of mutations in Wnt/β-catenin, hedgehog, Notch and PI3K pathways on GSK-3 activity-Diverse effects on cell growth, metabolism and cancer.Biochim Biophys Acta. 2016 Dec;1863(12):2942-2976. doi: 10.1016/j.bbamcr.2016.09.004. Epub 2016 Sep 6. Biochim Biophys Acta. 2016. PMID: 27612668 Review.
-
Crosstalk between Wnt/β-catenin and Hedgehog/Gli signaling pathways in colon cancer and implications for therapy.Cancer Biol Ther. 2015;16(1):1-7. doi: 10.4161/15384047.2014.972215. Cancer Biol Ther. 2015. PMID: 25692617 Free PMC article. Review.
Cited by
-
miRNA Expression Profile in Primary Limbal Epithelial Cells of Aniridia Patients.Invest Ophthalmol Vis Sci. 2025 Jan 2;66(1):20. doi: 10.1167/iovs.66.1.20. Invest Ophthalmol Vis Sci. 2025. PMID: 39786759 Free PMC article.
-
The Correlation Between Meibomian Gland Dysfunction and Aniridia-Associated Keratopathy: A Prospective Analysis.J Clin Med. 2025 Jan 27;14(3):828. doi: 10.3390/jcm14030828. J Clin Med. 2025. PMID: 39941499 Free PMC article.
References
-
- Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HH, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW. Identification of sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14(3):353–356. doi: 10.1038/ng1196-353. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

