Vaginal hormone-free moisturising cream is not inferior to an estriol cream for treating symptoms of vulvovaginal atrophy: Prospective, randomised study
- PMID: 35551533
- PMCID: PMC9098008
- DOI: 10.1371/journal.pone.0266633
Vaginal hormone-free moisturising cream is not inferior to an estriol cream for treating symptoms of vulvovaginal atrophy: Prospective, randomised study
Abstract
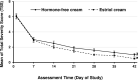
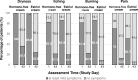
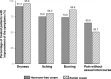
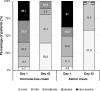
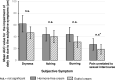
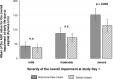
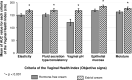
This prospective, open-label, multicentre, multinational, randomised trial investigated the non-inferiority of treatment with a vaginal hormone-free moisturising cream compared to a vaginal estriol (0.1%) cream in a panel of post-menopausal women suffering from symptoms of vulvovaginal dryness in a parallel group design. In total, 172 post-menopausal women were randomly allocated to either one of the two treatments, each administered for 43 days. The primary endpoint was the total severity score of subjective symptoms (dryness, itching, burning and pain unrelated to sexual intercourse) of the respective treatment period. Secondary endpoints were severity of single subjective symptoms (including dyspareunia if sexually active), impairment of daily life, Vaginal Health Index, as well as assessment of safety. In both groups, women treated with hormone-free moisturising cream and those treated with estriol cream, total severity score improved significantly compared to baseline by 5.0 (from 6.1 to 1.1) and by 5.4 (from 6.0 to 0.6), respectively, after 43 days of treatment (p < 0.0001). One-sided test of baseline differences (for a clinically relevant difference Δ = 1.5) confirmed the hormone-free moisturising cream to be non-inferior to the estriol cream. Severity of dyspareunia as well as impairment of daily life due to subjective symptoms, significantly improved for both treatment groups (p<0.0001). Subgroup analysis of women with mild or moderate impairment of daily life at baseline caused by "vaginal dryness" symptoms benefited from both creams, while women with severe impairment showed a significantly greater benefit from the estriol cream (p = 0.0032). Both treatments were well tolerated with no serious adverse events occurring. This study provides clinical evidence that a hormone-free vaginal moisturising cream cannot only improve vaginal dryness compared to an 0.1% estriol cream but also can relieve dyspareunia as well as improve woman's impairment of daily life, justifying its use as a first choice for mild or moderate vulvovaginal dryness symptoms.
Conflict of interest statement
The authors have read the journal’s policy. SGdA, LG, MH, and CM, have the following competing interest: They work for the company Dr. August Wolff GmbH & Co. KG Arzneimittel (Bielefeld, Germany) which is interested in developing products regarding treatment of vulvovaginal atrophy/ vaginal dryness symptoms. The principal investigator of the study [PS] as well as the statistical specialist [TWM] were also sponsored by Dr. August Wolff GmbH & Co. KG Arzneimittel. This does not alter the authors adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Portman DJ, Gass ML, Vulvovaginal Atrophy Terminology Consensus Conference P. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–8. doi: 10.1097/GME.0000000000000329 . - DOI - PubMed
-
- Castelo-Branco C, Biglia N, Nappi RE, Schwenkhagen A, Palacios S. Characteristics of post-menopausal women with genitourinary syndrome of menopause: Implications for vulvovaginal atrophy diagnosis and treatment selection. Maturitas. 2015;81(4):462–9. doi: 10.1016/j.maturitas.2015.05.007 Epub 2015 May 30. . - DOI - PubMed

