Clinical and biochemical endpoints and predictors of response to plasma exchange in septic shock: results from a randomized controlled trial
- PMID: 35551628
- PMCID: PMC9097091
- DOI: 10.1186/s13054-022-04003-2
Clinical and biochemical endpoints and predictors of response to plasma exchange in septic shock: results from a randomized controlled trial
Abstract
Background: Recently, a randomized controlled trial (RCT) demonstrated rapid but individually variable hemodynamic improvement with therapeutic plasma exchange (TPE) in patients with septic shock. Prediction of clinical efficacy in specific sepsis treatments is fundamental for individualized sepsis therapy.
Methods: In the original RCT, patients with septic shock of < 24 h duration and norepinephrine (NE) requirement ≥ 0.4 μg/kg/min received standard of care (SOC) or SOC + one single TPE. Here, we report all clinical and biological endpoints of this study. Multivariate mixed-effects modeling of NE reduction was performed to investigate characteristics that could be associated with clinical response to TPE.
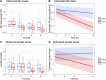
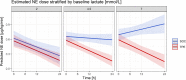
Results: A continuous effect of TPE on the reduction in NE doses over the initial 24 h was observed (SOC group: estimated NE dose reduction of 0.005 µg/kg/min per hour; TPE group: 0.018 µg/kg/min per hour, p = 0.004). Similarly, under TPE, serum lactate levels, continuously decreased over the initial 24 h in the TPE group, whereas lactate levels increased under SOC (p = 0.001). A reduction in biomarkers and disease mediators (such as PCT (p = 0.037), vWF:Ag (p < 0.001), Angpt-2 (p = 0.009), sTie-2 (p = 0.005)) along with a repletion of exhausted protective factors (such as AT-III (p = 0.026), Protein C (p = 0.012), ADAMTS-13 (p = 0.008)) could be observed in the TPE but not in the SOC group. In a multivariate mixed effects model, increasing baseline lactate levels led to greater NE dose reduction effects with TPE as opposed to SOC (p = 0.004).
Conclusions: Adjunctive TPE is associated with the removal of injurious mediators and repletion of consumed protective factors altogether leading to preserved hemodynamic stabilization in refractory septic shock. We identified that baseline lactate concentration as a potential response predictor might guide future designing of large RCTs that will further evaluate TPE with regard to hard endpoints. Trial registration Retrospectively registered 18th January 2020 at clinicaltrials.gov (Identifier: NCT04231994 ).
Keywords: Blood purification; Endothelium; Extracorporeal treatment[; Fresh frozen plasma; Personalized medicine; Plasmapheresis; Precision medicine; Sepsis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Effect of therapeutic plasma exchange on tissue factor and tissue factor pathway inhibitor in septic shock.Crit Care. 2024 Oct 30;28(1):351. doi: 10.1186/s13054-024-05142-4. Crit Care. 2024. PMID: 39478586 Free PMC article.
-
EXCHANGE-2: investigating the efficacy of add-on plasma exchange as an adjunctive strategy against septic shock-a study protocol for a randomized, prospective, multicenter, open-label, controlled, parallel-group trial.Trials. 2023 Apr 15;24(1):277. doi: 10.1186/s13063-023-07300-5. Trials. 2023. PMID: 37061693 Free PMC article.
-
The effect of therapeutic plasma exchange on the inflammatory response in septic shock: a secondary analysis of the EXCHANGE-1 trial.Intensive Care Med Exp. 2025 Feb 14;13(1):18. doi: 10.1186/s40635-025-00725-z. Intensive Care Med Exp. 2025. PMID: 39951217 Free PMC article.
-
[Extracorporeal Strategies in Sepsis Treatment: Role of Therapeutic Plasma Exchange].Anasthesiol Intensivmed Notfallmed Schmerzther. 2021 Feb;56(2):101-110. doi: 10.1055/a-1105-0572. Epub 2021 Feb 19. Anasthesiol Intensivmed Notfallmed Schmerzther. 2021. PMID: 33607671 Review. German.
-
Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012.Crit Care Med. 2013 Feb;41(2):580-637. doi: 10.1097/CCM.0b013e31827e83af. Crit Care Med. 2013. PMID: 23353941
Cited by
-
Effect of therapeutic plasma exchange on tissue factor and tissue factor pathway inhibitor in septic shock.Crit Care. 2024 Oct 30;28(1):351. doi: 10.1186/s13054-024-05142-4. Crit Care. 2024. PMID: 39478586 Free PMC article.
-
Development of a nomogram to predict 30-day mortality of sepsis patients with gastrointestinal bleeding: An analysis of the MIMIC-IV database.Heliyon. 2024 Feb 17;10(4):e26185. doi: 10.1016/j.heliyon.2024.e26185. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38404864 Free PMC article.
-
Perioperative Profiling of a Disintegrin and Metalloprotease with Thrombospondin Type 1 Motif, Member 13 (ADAMTS13) Activity in Cardiac Surgery: Kinetics and Mechanistic Insights.J Clin Med. 2025 Jul 11;14(14):4936. doi: 10.3390/jcm14144936. J Clin Med. 2025. PMID: 40725629 Free PMC article.
-
A few words of caution on blood purification in sepsis.Crit Care. 2025 Jan 25;29(1):45. doi: 10.1186/s13054-025-05268-z. Crit Care. 2025. PMID: 39856722 Free PMC article. No abstract available.
-
Influence of therapeutic plasma exchange treatment on short-term mortality of critically ill adult patients with sepsis-induced organ dysfunction: a systematic review and meta-analysis.Crit Care. 2024 Jan 4;28(1):12. doi: 10.1186/s13054-023-04795-x. Crit Care. 2024. PMID: 38178170 Free PMC article.
References
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. - PubMed
-
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. - PubMed
-
- Steinhagen F, Schmidt SV, Schewe JC, Peukert K, Klinman DM, Bode C. Immunotherapy in sepsis - brake or accelerate? Pharmacol Ther. 2020;208:107476. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

