IBRORS-MCL study: a Spanish retrospective and observational study of relapsed/refractory mantle-cell lymphoma treated with ibrutinib in routine clinical practice
- PMID: 35551632
- PMCID: PMC9392694
- DOI: 10.1007/s12185-022-03367-z
IBRORS-MCL study: a Spanish retrospective and observational study of relapsed/refractory mantle-cell lymphoma treated with ibrutinib in routine clinical practice
Abstract
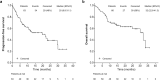
This retrospective study evaluated 66 patients diagnosed with relapsed and/or refractory mantle cell lymphoma (R/R MCL) treated with ibrutinib in Spain in routine clinical practice. At diagnosis, patients had a median age of 64.5 years, 63.6% presented with intermediate/high sMIPI (simplified prognostic index for advanced-stage mantle cell lymphoma), 24.5% had the blastoid variant, and 55.6% had a Ki67 > 30%. Patients had received a median of 2 prior lines of therapy (range 1-2; min-max 1-7). Overall response rate was 63.5%, with 38.1% of patients achieving complete response (CR). With a median duration of ibrutinib exposure of 10.7 months (range 5.2-19.6; min-max 0.3-36), the median progression-free survival (PFS) and overall survival (OS) were 20 months [95% confidence interval (CI) 8.8-31.1] and 32 months (95% CI 22.6-41.3), respectively, and were not reached in patients achieving CR. No grade ≥ 3 cardiovascular toxicity or bleeding was reported. This study supports that treatment with ibrutinib leads to high response rates and favorable survival outcomes in patients with R/R MCL.
Keywords: Clinical practice; Ibrutinib; Mantle-cell lymphoma; Real-world evidence; Relapsed/refractory.
© 2022. The Author(s).
Conflict of interest statement
JM Sancho: speaker fees for educational events from Roche, Janssen, Gilead-Kite, BMS-Celgene, Novartis, Takeda and Servier; Advisory boards for Roche, Janssen, Gilead-Kite, BMS-Celgene, Novartis, Incyte, Beigene and Lilly. A Marín Niebla: honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen, Takeda, Kiowa Kirin, Gilead, Lilly, Abbvie; Support for attending meetings and/or travel from Janssen, Takeda, Kiowa Kirin. FJ Capote: honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen, Takeda, Roche, BMS, Celgene, Amgen; Support for attending meetings and/or travel from Janssen, Takeda, Roche, BMS, Celgene, Amgen; advisory board for Janssen, Takeda, Roche, BMS, Celgene, Amgen; C Grande: advisory boards, symposia and attending conferences from Janssen. I Zeberio: honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS, Gilead, Novartis, Roche; Support for attending meetings and/or travel from BMS, Gilead, Janssen, Novartis, Roche, Incyte; advisory boards for BMS, Gilead, Janssen, Novartis, Roche, Incyte. JM Puerta: support for attending meetings from Novartis, Roche, Abbvie, Janssen, Astrazeneca, Gilead; Advisory boards for Roche, Novartis, Janssen, BMS; Financial support for research from Novartis, Roche, Pfizer. A Rivas: support for attending meetings and travel from Janssen, Kite and Takeda. E Pérez Ceballos: advisory boards for Takeda, Janssen, Incyte; honoraria for presentations from Takeda and Roche. A Vale: honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbvie, Janssen, Astrazeneca. A Martín García-Sancho: consulting fees from Roche, Celgene/BMS, Morphosys, Kyowa Kirin, Clinigen, Eusa Pharma, Novartis, Gilead, Servier, Incyte; honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Roche, Celgene/BMS, Janssen, Servier, Gilead, Takeda, EUSAPharma; Payment for expert testimony from Gilead, Roche; Support for attending meetings and/or travel from Roche, Servier, Celgene, Janssen, Kern Pharma. A Salar: Grants or contracts from Gilead; Consulting fees from Incyte and Beigene; honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Roche, EUSA Pharma, Janssen, BMS/Celgene. E González Barca: Consultancy from Janssen, Abbvie, Gilead, Kiowa, EUSA Pharma, Incyte, Lilly, Beigene, Novartis; Speaker fees: Janssen, Abbvie, Takeda, Roche, EUSA Pharma, Incyte; Travel grants: Janssen, Abbvie, Roche, EUSA Pharma. C. Pastoriza: support for attending meetings and/or travel from Janssen, Amgen and Abbvie. D. Conde-Royo: speaker fees from Janssen, Abbvie, Takeda. J Sánchez-García: honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Roche, Janssen, Abbvie, Pfizer. C Barrenetxea: honoraria for lecture fees from Janssen, Alexion and Takeda, and educational events from GSK. R Arranz: advisory Boards for Takeda and EUSA Pharma. JA Hernández-Rivas: grants or contracts from BMS-Celgene, Sanofi; Honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen, Roche, Abbvie, AstraZeneca, Lilly, Gilead, BMS-Celgene, Amgen, Takeda; advisory board for Janssen, Roche, Abbvie, AstraZeneca, Beigene, Lilly, Gilead, BMS-Celgene, Amgen, Takeda, Jazz Pharmaceuticals, Rovi. A Jiménez and E Rubio-Azpeitia are employees of Janssen. S Fernández, C Cañigral, E Donato, A Teruel and MJ Ramirez have nothing to disclosure.
Figures


References
-
- Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, Thieblemont C, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388(10044):565–575. doi: 10.1016/S0140-6736(16)00739-X. - DOI - PubMed

