Internet of things-based home noninvasive ventilation in COPD patients with hypercapnic chronic respiratory failure: study protocol for a randomized controlled trial
- PMID: 35551646
- PMCID: PMC9097410
- DOI: 10.1186/s13063-022-06372-z
Internet of things-based home noninvasive ventilation in COPD patients with hypercapnic chronic respiratory failure: study protocol for a randomized controlled trial
Abstract
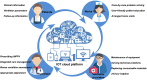
Background: Home noninvasive positive pressure ventilation (NIPPV) has become evidence-based care for stable hypercapnic chronic obstructive pulmonary disease (COPD) patients. There are still other challenges including appropriate follow-up, telemonitor, and management to ensure treatment effectiveness, compliance, and security and to improve quality of life. The Internet of things (IOT) is the name given to the network of devices and other "things" with built-in sensors, software, electronics, and network connectivity, communicating these objects over wireless networks and sending data to a cloud platform. The study aims to evaluate the effectiveness and safety of the IOT-based management of NIPPV for the COPD patients with hypercapnic chronic respiratory failure.
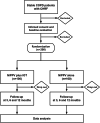
Methods: This multicenter, prospective, randomized controlled trial was conducted with a total of 200 COPD patients with chronic hypercapnic respiratory failure. Using a computer-generated randomization process, patients were randomized (in a 1:1 ratio) into the usual NIPPV (control group) or to receive additional IOT-based management (intervention group) for 12 months. The primary outcome was the Severe Respiratory Insufficiency (SRI) questionnaire. Secondary outcomes included compliance with the ventilator, gas exchange, lung function, health-related quality of life, hospitalization frequency, time to death within 1-year, all-cause mortality, safety analysis, and cost-effectiveness analysis.
Discussion: This study will be the first and largest randomized trial in China to evaluate the effectiveness and safety of the IOT-based management of NIPPV for COPD patients with chronic hypercapnic respiratory failure. The results will help to understand the current situation of IOT-based home ventilation and may provide new evidence for home NIPPV treatment and management in the future.
Trial registration: Chinese Clinical Trials Registry ChiCTR1800019536 . Registered on 17 November 2018.
Keywords: Chronic obstructive pulmonary disease; Follow-up; Hypercapnia respiratory failure; Internet of things; Management; Noninvasive positive pressure ventilation; Tele-monitoring.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–82. doi: 10.1164/rccm.201701-0218PP. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

