Carriage prevalence and genomic epidemiology of Staphylococcus aureus among Native American children and adults in the Southwestern USA
- PMID: 35551692
- PMCID: PMC9465076
- DOI: 10.1099/mgen.0.000806
Carriage prevalence and genomic epidemiology of Staphylococcus aureus among Native American children and adults in the Southwestern USA
Abstract
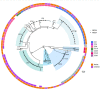
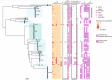
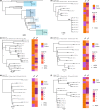
Native American individuals in the Southwestern USA experience a higher burden of invasive Staphylococcus aureus disease than the general population. However, little is known about S. aureus carriage in these communities. A cross-sectional study was conducted to determine the carriage prevalence, risk factors and genomic epidemiology of S. aureus among Native American children (<5 years, n=121) and adults (≥18 years, n=167) in the Southwestern USA. Short- and long-read sequencing data were generated using Illumina and Oxford Nanopore Technology platforms to produce high-quality hybrid assemblies, and antibiotic-resistance, virulence and pangenome analyses were performed. S. aureus carriage prevalence was 20.7 % among children, 30.2 % among adults 18-64 years and 16.7 % among adults ≥65 years. Risk factors among adults included recent surgery, prior S. aureus infection among household members, and recent use of gyms or locker rooms by household members. No risk factors were identified among children. The bacterial population structure was dominated by clonal complex 1 (CC1) (21.1 %), CC5 (22.2 %) and CC8 (22.2 %). Isolates from children and adults were intermixed throughout the phylogeny. While the S. aureus population was diverse, the carriage prevalence was comparable to that in the general USA population. Genomic and risk-factor data suggest household, community and healthcare transmission are important components of the local epidemiology.
Keywords: Native American; Staphylococcus aureus; carriage; genome epidemiology; phylogeny.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Centers for Disease Control and Prevention Atlanta, GA: CDC; 2014. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Neisseria meningitidis .www.cdc.gov/abcs/reports-findings/survreports/mrsa14.html
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

