The serotype a-EmaA adhesin of Aggregatibacter actinomycetemcomitans does not require O-PS synthesis for collagen binding activity
- PMID: 35551696
- PMCID: PMC10233464
- DOI: 10.1099/mic.0.001191
The serotype a-EmaA adhesin of Aggregatibacter actinomycetemcomitans does not require O-PS synthesis for collagen binding activity
Abstract
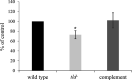
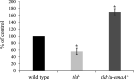
Aggregatibacter actinomycetemcomitans, a causative agent of periodontitis and non-oral diseases, synthesizes a trimeric extracellular matrix protein adhesin A (EmaA) that mediates collagen binding and biofilm formation. EmaA is found as two molecular forms, which correlate with the serotype of the bacterium. The canonical protein (b-EmaA), associated with serotypes b and c, has a monomeric molecular mass of 202 kDa. The collagen binding activity of b-EmaA is dependent on the presence of O-polysaccharide (O-PS), whereas biofilm activity is independent of O-PS synthesis. The EmaA associated with serotype a strains (a-EmaA) has a monomeric molecular mass of 173 kDa and differs in the amino acid sequence of the functional domain of the protein. In this study, a-emaA was confirmed to encode a protein that forms antenna-like appendages on the surface of the bacterium, which were found to be important for both collagen binding and biofilm formation. In an O-PS-deficient talose biosynthetic (tld) mutant strain, the electrophoretic mobility of the a-EmaA monomers was altered and the amount of membrane-associated EmaA was decreased when compared to the parent strain. The mass of biofilm formed remained unchanged. Interestingly, the collagen binding activity of the mutant strain was similar to the activity associated with the parent strain, which differs from that observed with the canonical b-EmaA isoform. These data suggest that the properties of the a-EmaA isoform are like those of b-EmaA, with the exception that collagen binding activity is independent of the presence or absence of the O-PS.
Keywords: Gram-negative bacterium; O-polysaccharides; adhesin; collagen; endocarditis; glycosylation; periodontal disease.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


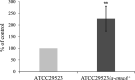

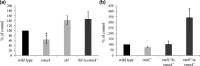
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

