Spatiotemporal Distribution of Electrically Evoked Spinal Compound Action Potentials During Spinal Cord Stimulation
- PMID: 35551869
- PMCID: PMC9643656
- DOI: 10.1016/j.neurom.2022.03.007
Spatiotemporal Distribution of Electrically Evoked Spinal Compound Action Potentials During Spinal Cord Stimulation
Abstract
Objectives: Recent studies using epidural spinal cord stimulation (SCS) have demonstrated restoration of motor function in individuals previously diagnosed with chronic spinal cord injury (SCI). In parallel, the spinal evoked compound action potentials (ECAPs) induced by SCS have been used to gain insight into the mechanisms of SCS-based chronic pain therapy and to titrate closed-loop delivery of stimulation. However, the previous characterization of ECAPs recorded during SCS was performed with one-dimensional, cylindrical electrode leads. Herein, we describe the unique spatiotemporal distribution of ECAPs induced by SCS across the medial-lateral and rostral-caudal axes of the spinal cord, and their relationship to polysynaptic lower-extremity motor activation.
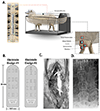
Materials and methods: In each of four sheep, two 24-contact epidural SCS arrays were placed on the lumbosacral spinal cord, spanning the L3 to L6 vertebrae. Spinal ECAPs were recorded during SCS from nonstimulating contacts of the epidural arrays, which were synchronized to bilateral electromyography (EMG) recordings from six back and lower-extremity muscles.
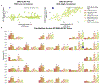
Results: We observed a triphasic P1, N1, P2 peak morphology and propagation in the ECAPs during midline and lateral stimulation. Distinct regions of lateral stimulation resulted in simultaneously increased ECAP and EMG responses compared with stimulation at adjacent lateral contacts. Although EMG responses decreased during repetitive stimulation bursts, spinal ECAP amplitude did not significantly change. Both spinal ECAP responses and EMG responses demonstrated preferential ipsilateral recruitment during lateral stimulation compared with midline stimulation. Furthermore, EMG responses were correlated with stimulation that resulted in increased ECAP amplitude on the ipsilateral side of the electrode array.
Conclusions: These results suggest that ECAPs can be used to investigate the effects of SCS on spinal sensorimotor networks and to inform stimulation strategies that optimize the clinical benefit of SCS in the context of managing chronic pain and the restoration of sensorimotor function after SCI.
Keywords: Chronic pain; electromyography; functional restoration; neurostimulation; spinal cord injury; spinal cord stimulation.
Published by Elsevier Inc.
Conflict of interest statement
Figures
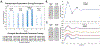





References
-
- Linderoth B, Spinal cord stimulation: A brief update on mechanisms of action, Eur. J. Pain Suppl 3 (2009). 10.1016/j.eujps.2009.07.003. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

