LncRNA CCAT1 enhances chemoresistance in hepatocellular carcinoma by targeting QKI-5
- PMID: 35552451
- PMCID: PMC9098857
- DOI: 10.1038/s41598-022-11644-4
LncRNA CCAT1 enhances chemoresistance in hepatocellular carcinoma by targeting QKI-5
Abstract
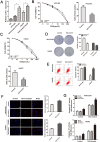
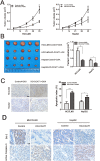
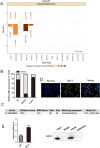
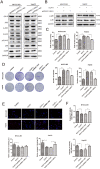
A major reason for treatment failure of cancer is acquisition of drug resistance. The specific mechanisms underlying hepatocellular carcinoma (HCC) chemoresistance need to be fully elucidated. lncRNAs involve in drug resistance in some cancers, however, the exact functions of lncRNA colon cancer-associated transcript 1 (CCAT1) in oxaliplatin resistance in HCC are still unknown. Our study indicated that CCAT1 promoted HCC proliferation and reduced the apoptosis induced by oxaliplatin. Knockout of CCAT1 could increased chemosensitivity in vitro and in vivo. Further study found that QKI-5 was an important mediator and blocking of QKI-5/p38 MAPK signaling pathway could enhance oxaliplatin sensitivity. In conclusions, CCAT1 promoted proliferation and oxaliplatin resistance via QKI-5/p38 MAPK signaling pathway in HCC. Targeting CCAT1 in combination with chemotherapeutics may be a promising alternative to reverse drug resistance in HCC treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Long noncoding RNA LEF1-AS1 acts as a microRNA-10a-5p regulator to enhance MSI1 expression and promote chemoresistance in hepatocellular carcinoma cells through activating AKT signaling pathway.J Cell Biochem. 2021 Jan;122(1):86-99. doi: 10.1002/jcb.29833. Epub 2020 Aug 12. J Cell Biochem. 2021. PMID: 32786108
-
LSD1-Demethylated LINC01134 Confers Oxaliplatin Resistance Through SP1-Induced p62 Transcription in HCC.Hepatology. 2021 Dec;74(6):3213-3234. doi: 10.1002/hep.32079. Epub 2021 Oct 5. Hepatology. 2021. PMID: 34322883
-
LncRNA PCGEM1 mediates oxaliplatin resistance in hepatocellular carcinoma via miR-129-5p/ETV1 axis in vitro.Adv Clin Exp Med. 2021 Aug;30(8):831-838. doi: 10.17219/acem/135533. Adv Clin Exp Med. 2021. PMID: 34286514
-
Non-coding RNA in drug resistance of hepatocellular carcinoma.Biosci Rep. 2018 Oct 9;38(5):BSR20180915. doi: 10.1042/BSR20180915. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30224380 Free PMC article. Review.
-
The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma.Mol Cancer. 2019 Oct 25;18(1):147. doi: 10.1186/s12943-019-1086-z. Mol Cancer. 2019. PMID: 31651347 Free PMC article. Review.
Cited by
-
RNA-binding protein quaking: a multifunctional regulator in tumour progression.Ann Med. 2025 Dec;57(1):2443046. doi: 10.1080/07853890.2024.2443046. Epub 2024 Dec 23. Ann Med. 2025. PMID: 39711373 Free PMC article. Review.
-
The interplay between noncoding RNAs and drug resistance in hepatocellular carcinoma: the big impact of little things.J Transl Med. 2023 Jun 7;21(1):369. doi: 10.1186/s12967-023-04238-9. J Transl Med. 2023. PMID: 37286982 Free PMC article. Review.
-
A novel disulfidptosis-related lncRNA signature for predicting prognosis and potential targeted therapy in hepatocellular carcinoma.Medicine (Baltimore). 2024 Jan 26;103(4):e36513. doi: 10.1097/MD.0000000000036513. Medicine (Baltimore). 2024. PMID: 38277541 Free PMC article.
-
Deciphering STAT3 signaling potential in hepatocellular carcinoma: tumorigenesis, treatment resistance, and pharmacological significance.Cell Mol Biol Lett. 2023 Apr 21;28(1):33. doi: 10.1186/s11658-023-00438-9. Cell Mol Biol Lett. 2023. PMID: 37085753 Free PMC article. Review.
-
Functional Relevance of the Long Intergenic Non-Coding RNA Regulator of Reprogramming (Linc-ROR) in Cancer Proliferation, Metastasis, and Drug Resistance.Noncoding RNA. 2023 Jan 31;9(1):12. doi: 10.3390/ncrna9010012. Noncoding RNA. 2023. PMID: 36827545 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

