Impaired Hematopoietic Stem/Progenitor Cell Traffic and Multi-organ Damage in Diabetes
- PMID: 35552468
- PMCID: PMC9406601
- DOI: 10.1093/stmcls/sxac035
Impaired Hematopoietic Stem/Progenitor Cell Traffic and Multi-organ Damage in Diabetes
Abstract
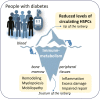
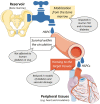
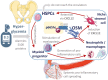
During antenatal development, hematopoietic stem/progenitor cells (HSPCs) arise from a specialized endothelium and migrate from the extraembryonic mesoderm to the fetal liver before establishing hematopoiesis in the bone marrow (BM). It is still debated whether, in adulthood, HSPCs display such ontologic overlap with vascular cells and capacity for endothelial differentiation. Yet, adult HSPCs retain a prominent migratory activity and traffic in the bloodstream to secondary lymphoid organs and all peripheral tissues, before eventually returning to the BM. While patrolling parenchymatous organs, HSPCs locate close to the vasculature, where they establish local hematopoietic islands and contribute to tissue homeostasis by paracrine signals. Solid evidence shows that diabetes mellitus jeopardizes the traffic of HSPCs from BM to the circulation and peripheral tissues, a condition called "mobilopathy." A reduction in the levels of circulating HSPCs is the most immediate and apparent consequence, which has been consistently observed in human diabetes, and is strongly associated with future risk for multi-organ damage, including micro- and macro-angiopathy. But the shortage of HSPCs in the blood is only the visible tip of the iceberg. Abnormal HSPC traffic results from a complex interplay among metabolism, innate immunity, and hematopoiesis. Notably, mobilopathy is mechanistically connected with diabetes-induced myelopoiesis. Impaired traffic of HSPCs and enhanced generation of pro-inflammatory cells synergize for tissue damage and impair the resolution of inflammation. We herein summarize the current evidence that diabetes affects HSPC traffic, which are the causes and consequences of such alteration, and how it contributes to the overall disease burden.
Keywords: CD34+; adult hematopoietic stem cells; bone marrow; diabetes; mobilization.
© The Author(s) 2022. Published by Oxford University Press.
Figures

Similar articles
-
Diabetes-Associated Myelopoiesis Drives Stem Cell Mobilopathy Through an OSM-p66Shc Signaling Pathway.Diabetes. 2019 Jun;68(6):1303-1314. doi: 10.2337/db19-0080. Epub 2019 Apr 1. Diabetes. 2019. PMID: 30936144
-
TGFBI Expressed by Bone Marrow Niche Cells and Hematopoietic Stem and Progenitor Cells Regulates Hematopoiesis.Stem Cells Dev. 2018 Nov 1;27(21):1494-1506. doi: 10.1089/scd.2018.0124. Epub 2018 Sep 6. Stem Cells Dev. 2018. PMID: 30084753 Free PMC article.
-
Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome.J Appl Physiol (1985). 2016 Mar 15;120(6):624-32. doi: 10.1152/japplphysiol.00925.2015. Epub 2016 Jan 7. J Appl Physiol (1985). 2016. PMID: 26744505
-
Parallels between immune driven-hematopoiesis and T cell activation: 3 signals that relay inflammatory stress to the bone marrow.Exp Cell Res. 2014 Dec 10;329(2):239-47. doi: 10.1016/j.yexcr.2014.09.016. Epub 2014 Sep 22. Exp Cell Res. 2014. PMID: 25246130 Review.
-
Concise Review: Perspectives and Clinical Implications of Bone Marrow and Circulating Stem Cell Defects in Diabetes.Stem Cells. 2017 Jan;35(1):106-116. doi: 10.1002/stem.2445. Epub 2016 Jul 11. Stem Cells. 2017. PMID: 27401837 Review.
Cited by
-
Putative circulating adipose tissue-derived stem cells, obesity, and metabolic syndrome features.J Endocrinol Invest. 2023 Oct;46(10):2147-2155. doi: 10.1007/s40618-023-02067-7. Epub 2023 Mar 23. J Endocrinol Invest. 2023. PMID: 36952215 Free PMC article.
-
Cardiovascular complications of diabetes: role of non-coding RNAs in the crosstalk between immune and cardiovascular systems.Cardiovasc Diabetol. 2023 May 24;22(1):122. doi: 10.1186/s12933-023-01842-3. Cardiovasc Diabetol. 2023. PMID: 37226245 Free PMC article. Review.
-
NOD1 deficiency ameliorates the progression of diabetic retinopathy by modulating bone marrow-retina crosstalk.Stem Cell Res Ther. 2024 Feb 9;15(1):38. doi: 10.1186/s13287-024-03654-y. Stem Cell Res Ther. 2024. PMID: 38336763 Free PMC article.
-
Vitamin D and Cardiovascular Risk: Stemming the Tide of Poor Outcomes in Coronary Heart Disease.JACC Adv. 2024 Jan 10;3(2):100803. doi: 10.1016/j.jacadv.2023.100803. eCollection 2024 Feb. JACC Adv. 2024. PMID: 38939406 Free PMC article.
-
The cardio-renal-metabolic connection: a review of the evidence.Cardiovasc Diabetol. 2023 Jul 31;22(1):195. doi: 10.1186/s12933-023-01937-x. Cardiovasc Diabetol. 2023. PMID: 37525273 Free PMC article. Review.
References
-
- Richman CM, Weiner RS, Yankee RA.. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47(6):1031-1039. - PubMed
-
- Vermeulen M, Le Pesteur F, Gagnerault MC, et al. . Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92(3):894-900. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

