Single unit analysis and wide-field imaging reveal alterations in excitatory and inhibitory neurons in glioma
- PMID: 35552612
- PMCID: PMC10202150
- DOI: 10.1093/brain/awac168
Single unit analysis and wide-field imaging reveal alterations in excitatory and inhibitory neurons in glioma
Abstract
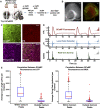
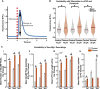
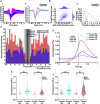
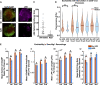
While several studies have attributed the development of tumour-associated seizures to an excitatory-inhibitory imbalance, we have yet to resolve the spatiotemporal interplay between different types of neuron in glioma-infiltrated cortex. Herein, we combined methods for single unit analysis of microelectrode array recordings with wide-field optical mapping of Thy1-GCaMP pyramidal cells in an ex vivo acute slice model of diffusely infiltrating glioma. This enabled simultaneous tracking of individual neurons from both excitatory and inhibitory populations throughout seizure-like events. Moreover, our approach allowed for observation of how the crosstalk between these neurons varied spatially, as we recorded across an extended region of glioma-infiltrated cortex. In tumour-bearing slices, we observed marked alterations in single units classified as putative fast-spiking interneurons, including reduced firing, activity concentrated within excitatory bursts and deficits in local inhibition. These results were correlated with increases in overall excitability. Mechanistic perturbation of this system with the mTOR inhibitor AZD8055 revealed increased firing of putative fast-spiking interneurons and restoration of local inhibition, with concomitant decreases in overall excitability. Altogether, our findings suggest that diffusely infiltrating glioma affect the interplay between excitatory and inhibitory neuronal populations in a reversible manner, highlighting a prominent role for functional mechanisms linked to mTOR activation.
Keywords: glioma; mTOR; single unit; tumour-associated seizures.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures

Comment in
-
Exciting insights into tumour-associated epilepsy with electrophysiological and optical recording.Brain. 2022 Oct 21;145(10):3345-3346. doi: 10.1093/brain/awac351. Brain. 2022. PMID: 36148603 No abstract available.
References
-
- Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia. 2013;54:12–17. - PubMed
-
- Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J. Extracellular glutamate and other metabolites in and around RG2 rat glioma: An intracerebral microdialysis study. J Neurooncol. 2000;47:11–22. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

