Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2- ABC: Updated Exploratory Analyses of PALOMA-3, a Double-blind, Phase III Randomized Study
- PMID: 35552673
- PMCID: PMC9662922
- DOI: 10.1158/1078-0432.CCR-22-0305
Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2- ABC: Updated Exploratory Analyses of PALOMA-3, a Double-blind, Phase III Randomized Study
Abstract
Purpose: To conduct an updated exploratory analysis of overall survival (OS) with a longer median follow-up of 73.3 months and evaluate the prognostic value of molecular analysis by circulating tumor DNA (ctDNA).
Patients and methods: Patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced breast cancer (ABC) were randomized 2:1 to receive palbociclib (125 mg orally/day; 3/1 week schedule) and fulvestrant (500 mg intramuscularly) or placebo and fulvestrant. This OS analysis was performed when 75% of enrolled patients died (393 events in 521 randomized patients). ctDNA analysis was performed among patients who provided consent.
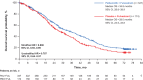
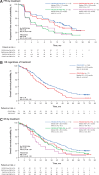
Results: At the data cutoff (August 17, 2020), 258 and 135 deaths occurred in the palbociclib and placebo groups, respectively. The median OS [95% confidence interval (CI)] was 34.8 months (28.8-39.9) in the palbociclib group and 28.0 months (23.5-33.8) in the placebo group (stratified hazard ratio, 0.81; 95% CI, 0.65-0.99). The 6-year OS rate (95% CI) was 19.1% (14.9-23.7) and 12.9% (8.0-19.1) in the palbociclib and placebo groups, respectively. Favorable OS with palbociclib plus fulvestrant compared with placebo plus fulvestrant was observed in most subgroups, particularly in patients with endocrine-sensitive disease, no prior chemotherapy for ABC and low circulating tumor fraction and regardless of ESR1, PIK3CA, or TP53 mutation status. No new safety signals were identified.
Conclusions: The clinically meaningful improvement in OS associated with palbociclib plus fulvestrant was maintained with >6 years of follow-up in patients with HR+/HER2- ABC, supporting palbociclib plus fulvestrant as a standard of care in these patients.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 2015;21:4760–6. - PubMed
-
- Cristofanilli M, DeMichele A, Giorgetti C, Turner NC, Slamon DJ, Im SA, et al. Predictors of prolonged benefit from palbociclib plus fulvestrant in women with endocrine-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer in PALOMA-3. Eur J Cancer 2018;104:21–31. - PubMed
-
- Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–39. - PubMed
-
- Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018;379:1926–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

