Persistence of Chemotherapy-Induced Peripheral Neuropathy Despite Vincristine Reduction in Childhood B-Acute Lymphoblastic Leukemia
- PMID: 35552709
- PMCID: PMC9360458
- DOI: 10.1093/jnci/djac095
Persistence of Chemotherapy-Induced Peripheral Neuropathy Despite Vincristine Reduction in Childhood B-Acute Lymphoblastic Leukemia
Abstract
Background: Children with B-acute lymphoblastic leukemia (B-ALL) are at risk for chemotherapy-induced peripheral neuropathy (CIPN). Children's Oncology Group AALL0932 randomized reduction in vincristine and dexamethasone (every 4 weeks vs 12 weeks during maintenance in the average-risk subset of National Cancer Institute standard-B-ALL (SR AR B-ALL). We longitudinally measured CIPN, overall and by treatment group.
Methods: AALL0932 standard-B-ALL patients aged 3 years and older were evaluated at T1-T4 (end consolidation, maintenance month 1, maintenance month 18, 12 months posttherapy). Physical and occupational therapists (PT/OT) measured motor CIPN (hand and ankle strength, dorsiflexion and plantarflexion range of motion), sensory CIPN (finger and toe vibration and touch), function (dexterity [Purdue Pegboard], and walking efficiency [Six-Minute Walk]). Proxy-reported function (Pediatric Outcome Data Collection Instrument) and quality of life (Pediatric Quality of Life Inventory) were assessed. Age- and sex-matched z scores and proportion impaired were measured longitudinally and compared between groups.
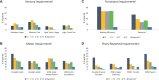
Results: Consent and data were obtained from 150 participants (mean age = 5.1 years [SD = 1.7], 48.7% female). Among participants with completed evaluations, 81.8% had CIPN at T1 (74.5% motor, 34.1% sensory). When examining severity of PT/OT outcomes, only handgrip strength (P < .001) and walking efficiency (P = .02) improved from T1-T4, and only dorsiflexion range of motion (46.7% vs 14.7%; P = .008) and handgrip strength (22.2% vs 37.1%; P = .03) differed in vincristine and dexamethasone every 4 weeks vs vincristine and dexamethasone 12 weeks at T4. Proxy-reported outcomes improved from T1 to T4 (P < .001), and most did not differ between groups.
Conclusions: CIPN is prevalent early in B-ALL therapy and persists at least 12 months posttherapy. Most outcomes did not differ between treatment groups despite reduction in vincristine frequency. Children with B-ALL should be monitored for CIPN, even with reduced vincristine frequency.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer.Cochrane Database Syst Rev. 2013 Apr 30;(4):CD008796. doi: 10.1002/14651858.CD008796.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Mar 31;3:CD008796. doi: 10.1002/14651858.CD008796.pub3. PMID: 23633361 Updated.
-
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer.Cochrane Database Syst Rev. 2016 Mar 31;3(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. Cochrane Database Syst Rev. 2016. PMID: 27030386 Free PMC article.
-
Exploring clinical markers of Axon degeneration processes in Chemotherapy-induced peripheral neuropathy among young adults receiving vincristine or paclitaxel.BMC Neurol. 2024 Sep 28;24(1):366. doi: 10.1186/s12883-024-03877-9. BMC Neurol. 2024. PMID: 39342135 Free PMC article.
-
Effect of Exercise on Chemotherapy-Induced Peripheral Neuropathy Among Patients Treated for Ovarian Cancer: A Secondary Analysis of a Randomized Clinical Trial.JAMA Netw Open. 2023 Aug 1;6(8):e2326463. doi: 10.1001/jamanetworkopen.2023.26463. JAMA Netw Open. 2023. PMID: 37526937 Free PMC article. Clinical Trial.
-
Hypothalamic-pituitary-adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia.Cochrane Database Syst Rev. 2017 Nov 6;11(11):CD008727. doi: 10.1002/14651858.CD008727.pub4. Cochrane Database Syst Rev. 2017. PMID: 29106702 Free PMC article.
Cited by
-
Parents' perception of treatment-related toxicity in children treated according to the NOPHO ALL2008 protocol for acute lymphoblastic leukemia.Hemasphere. 2024 Jul 12;8(7):e124. doi: 10.1002/hem3.124. eCollection 2024 Jul. Hemasphere. 2024. PMID: 39006374 Free PMC article.
-
Usability Evaluation of the Revised Color Me Healthy Symptom Assessment App: Perspectives of Children and Parents.Children (Basel). 2024 Oct 4;11(10):1215. doi: 10.3390/children11101215. Children (Basel). 2024. PMID: 39457180 Free PMC article.
-
Characterising vincristine-induced peripheral neuropathy in adults: symptom development and long-term persistent outcomes.Support Care Cancer. 2024 Apr 9;32(5):278. doi: 10.1007/s00520-024-08484-5. Support Care Cancer. 2024. PMID: 38592525 Free PMC article.
-
Transcriptome Profiling of miRNA-mRNA Interactions and Associated Mechanisms in Chemotherapy-Induced Neuropathic Pain.Mol Neurobiol. 2023 Oct;60(10):5672-5690. doi: 10.1007/s12035-023-03398-5. Epub 2023 Jun 19. Mol Neurobiol. 2023. PMID: 37332017
-
Effect of concurrent training on physical performance and quality of life in children with malignancy: A systematic review and meta-analysis.Front Public Health. 2023 Mar 17;11:1127255. doi: 10.3389/fpubh.2023.1127255. eCollection 2023. Front Public Health. 2023. PMID: 37006540 Free PMC article.
References
-
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A.. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83-103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

