ePHex: a phase 3, double-blind, placebo-controlled, randomized study to evaluate long-term efficacy and safety of Oxalobacter formigenes in patients with primary hyperoxaluria
- PMID: 35552824
- PMCID: PMC9763141
- DOI: 10.1007/s00467-022-05591-5
ePHex: a phase 3, double-blind, placebo-controlled, randomized study to evaluate long-term efficacy and safety of Oxalobacter formigenes in patients with primary hyperoxaluria
Abstract
Background: Primary hyperoxalurias (PHs) are rare genetic diseases that increase the endogenous level of oxalate, a waste metabolite excreted predominantly by the kidneys and also the gut. Treatments aim to improve oxalate excretion, or reduce oxalate generation, to prevent kidney function deterioration. Oxalobacter formigenes is an oxalate metabolizing bacterium. This Phase III, double-blind, placebo-controlled randomized trial investigated the effectiveness of orally administered Oxabact™, a lyophilized O. formigenes formulation, at reducing plasma oxalate levels in patients suffering from PH.
Methods: Subjects (≥ 2 years of age) with a diagnosis of PH and maintained but suboptimal kidney function (mean estimated glomerular filtration rate at baseline < 90 mL/min/1.73 m2) were eligible to participate. Subjects were randomized to receive Oxabact or placebo twice daily for 52 weeks. Change from baseline in plasma oxalate concentration at Week 52 was the primary study endpoint.
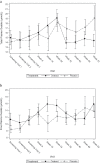
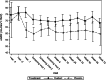
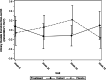
Results: Forty-three subjects were screened, 25 were recruited and one was discontinued. At Week 52, O. formigenes was established in the gut of subjects receiving Oxabact. Despite decreasing plasma oxalate level in subjects treated with Oxabact, and stable/increased levels with placebo, there was no significant difference between groups in the primary outcome (Least Squares mean estimate of treatment difference was - 3.80 μmol/L; 95% CI: - 7.83, 0.23; p-value = 0.064). Kidney function remained stable in both treatments.
Conclusions: Oxabact treatment may have stabilized/reduced plasma oxalate versus a rise with placebo, but the difference over 12 months was not statistically significant (p = 0.06). A subtle effect observed with Oxabact suggests that O. formigenes may aid in preventing kidney stones. A higher resolution version of the Graphical abstract is available as Supplementary information.
Keywords: Oxabact; Oxalate; Oxalobacter formigenes; Primary hyperoxaluria; eGFR.
© 2022. The Author(s).
Conflict of interest statement
Ana Banos, Bastian Dehmel and Elisabeth Lindner were employed by OxThera Intellectual Property AB, Stockholm, Sweden.
Figures

References
-
- Oxalosis & Hyperoxaluria Foundation (2019) https://www.ohf.org. Accessed 24 September 2021
-
- Milliner DS, Harris PC, Cogal AG, Lieske JC (2002) Primary hyperoxaluria type 1. 2002 Jun 19 [Updated 2017 Nov 30]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1283/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

