Efficacy and safety of N-acetyl-GED-0507-34-LEVO gel in patients with moderate-to severe facial acne vulgaris: a phase IIb randomized double-blind, vehicle-controlled trial
- PMID: 35553043
- PMCID: PMC9796277
- DOI: 10.1111/bjd.21663
Efficacy and safety of N-acetyl-GED-0507-34-LEVO gel in patients with moderate-to severe facial acne vulgaris: a phase IIb randomized double-blind, vehicle-controlled trial
Abstract
Background: Preliminary in vitro and in vivo studies have supported the efficacy of the peroxisome proliferator-activated receptor-γ (PPARγ) modulator N-acetyl-GED-0507-34-LEVO (NAC-GED) for the treatment of acne-inducing sebocyte differentiation, improving sebum composition and controlling the inflammatory process.
Objectives: To evaluate the efficacy and safety of NAC-GED (5% and 2%) in patients with moderate-to-severe facial acne vulgaris.
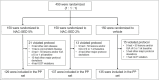
Methods: This double-blind phase II randomized controlled clinical trial was conducted at 36 sites in Germany, Italy and Poland. Patients aged 12-30 years with facial acne, an Investigator Global Assessment (IGA) score of 3-4, and an inflammatory and noninflammatory lesion count of 20-100 were randomized to topical application of the study drug (2% or 5%) or placebo (vehicle), once daily for 12 weeks. The co-primary efficacy endpoints were percentage change from baseline in total lesion count (TLC) and IGA success at week 12; the safety endpoints were adverse events (AEs) and serious AEs. This study was registered with EudraCT (2018-003307-19).
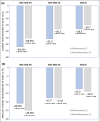
Results: Between Q1 in 2019 and Q1 in 2020 450 patients [n = 418 (92·9%) IGA 3; n = 32 (7·1%) IGA 4] were randomly assigned to NAC-GED 5% (n = 150), NAC-GED 2% (n = 150) or vehicle (n = 150). The percentage change in TLC reduction was statistically significantly higher in both the NAC-GED 5% [-57·1%, 95% confidence interval (CI) -60·8 to -53·4; P < 0·001] and NAC-GED 2% (-44·7%, 95% CI -49·1 to -40·1; P < 0·001) groups compared with vehicle (-33·9%, 95% CI -37·6 to -30·2). A higher proportion of patients treated with NAC-GED 5% experienced IGA success (45%, 95% CI 38-53) vs. the vehicle group (24%, 95% CI 18-31; P < 0·001). The IGA success rate was 33% in the NAC-GED 2% group (P = not significant vs. vehicle). The percentage of patients who had one or more AEs was 19%, 16% and 19% in the NAC-GED 5%, NAC-GED 2% and vehicle groups, respectively.
Conclusions: The topical application of NAC-GED 5% reduced TLC, increased the IGA success rate and was safe for use in patients with acne vulgaris. Thus, NAC-GED, a new PPARγ modulator, showed an effective clinical response. What is already known about this topic? Acne vulgaris, one of the most common dermatological diseases, affects more than 85% of adolescents. There is a medical need for innovative and safe treatment of acne vulgaris. The peroxisome proliferator-activated receptor-γ (PPARγ) is involved in lipid metabolism and specifically in cell differentiation, sebum production and the inflammatory reaction. What does this study add? N-acetyl-GED-0507-34-LEVO (NAC-GED 5%), a PPARγ modulator, significantly improves acne manifestations in patients with moderate-to-severe acne and is safe and well tolerated. The results suggest that the PPARγ receptor is a novel therapeutic target for acne. The results provide a basis for a large phase III trial to assess the effectiveness and safety profile of NAC-GED in combating a disease that afflicts 80-90% of adolescents.
© 2022 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Conflict of interest statement
M.P. was the designated scientific expert for the study; C.C.Z. was the designated clinical expert. The laboratories managed by M.P. received a research grant and C.C.Z. received fees for consultation from PPM Services SA. L.M., R.M. and A.N. are employees of the clinical research organizations (funded by PPM Services SA) in charge of the management, monitoring, data management and data analysis of the study.
Figures
Comment in
-
Advances in acne clinical research: a new class of topical treatment for moderate-to-severe facial acne.Br J Dermatol. 2022 Oct;187(4):455-456. doi: 10.1111/bjd.21711. Epub 2022 Jul 11. Br J Dermatol. 2022. PMID: 35818065 No abstract available.
References
-
- Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community‐based study. J Invest Dermatol 2009; 129:2136–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

