Seroconversion following COVID-19 vaccination: can we optimize protective response in CD20-treated individuals?
- PMID: 35553629
- PMCID: PMC9113152
- DOI: 10.1093/cei/uxab015
Seroconversion following COVID-19 vaccination: can we optimize protective response in CD20-treated individuals?
Abstract
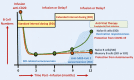
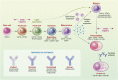
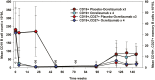
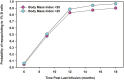
Although there is an ever-increasing number of disease-modifying treatments for relapsing multiple sclerosis (MS), few appear to influence coronavirus disease 2019 (COVID-19) severity. There is concern about the use of anti-CD20-depleting monoclonal antibodies, due to the apparent increased risk of severe disease following severe acute respiratory syndrome corona virus two (SARS-CoV-2) infection and inhibition of protective anti-COVID-19 vaccine responses. These antibodies are given as maintenance infusions/injections and cause persistent depletion of CD20+ B cells, notably memory B-cell populations that may be instrumental in the control of relapsing MS. However, they also continuously deplete immature and mature/naïve B cells that form the precursors for infection-protective antibody responses, thus blunting vaccine responses. Seroconversion and maintained SARS-CoV-2 neutralizing antibody levels provide protection from COVID-19. However, it is evident that poor seroconversion occurs in the majority of individuals following initial and booster COVID-19 vaccinations, based on standard 6 monthly dosing intervals. Seroconversion may be optimized in the anti-CD20-treated population by vaccinating prior to treatment onset or using extended/delayed interval dosing (3-6 month extension to dosing interval) in those established on therapy, with B-cell monitoring until (1-3%) B-cell repopulation occurs prior to vaccination. Some people will take more than a year to replete and therefore protection may depend on either the vaccine-induced T-cell responses that typically occur or may require prophylactic, or rapid post-infection therapeutic, antibody or small-molecule antiviral treatment to optimize protection against COVID-19. Further studies are warranted to demonstrate the safety and efficacy of such approaches and whether or not immunity wanes prematurely as has been observed in the other populations.
Keywords: CD20 B cells; COVID-19 vaccination; autoimmunity; immunotherapy; multiple sclerosis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Immunology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
A retrospective evaluation of seroconversion after COVID-19 during the early Omicron wave in fully vaccinated multiple sclerosis patients receiving anti-CD20 therapies.Mult Scler Relat Disord. 2023 Mar;71:104574. doi: 10.1016/j.msard.2023.104574. Epub 2023 Feb 15. Mult Scler Relat Disord. 2023. PMID: 36827874 Free PMC article.
-
Strong T-cell activation in response to COVID-19 vaccination in multiple sclerosis patients receiving B-cell depleting therapies.Front Immunol. 2022 Aug 5;13:926318. doi: 10.3389/fimmu.2022.926318. eCollection 2022. Front Immunol. 2022. PMID: 35990701 Free PMC article.
-
Three to four mRNA COVID-19 vaccines in multiple sclerosis patients on immunosuppressive drugs: Seroconversion and variant neutralization.Eur J Neurol. 2023 Sep;30(9):2781-2792. doi: 10.1111/ene.15925. Epub 2023 Jun 25. Eur J Neurol. 2023. PMID: 37310391
-
COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases.Clin Exp Immunol. 2020 Nov;202(2):149-161. doi: 10.1111/cei.13495. Epub 2020 Aug 1. Clin Exp Immunol. 2020. PMID: 32671831 Free PMC article. Review.
-
Impact of Therapy in Patients with Hematologic Malignancies on Seroconversion Rates After SARS-CoV-2 Vaccination.Oncologist. 2022 Apr 5;27(4):e357-e361. doi: 10.1093/oncolo/oyac032. Oncologist. 2022. PMID: 35274729 Free PMC article.
Cited by
-
B-cell repopulation dynamics and drug pharmacokinetics impact SARS-CoV-2 vaccine efficacy in anti-CD20-treated multiple sclerosis patients.Eur J Neurol. 2022 Nov;29(11):3317-3328. doi: 10.1111/ene.15492. Epub 2022 Jul 20. Eur J Neurol. 2022. PMID: 35808856 Free PMC article.
-
Ocrelizumab effect on humoral and cellular immunity in multiple sclerosis and its clinical correlates: a 3-year observational study.J Neurol. 2023 Jan;270(1):272-282. doi: 10.1007/s00415-022-11350-1. Epub 2022 Sep 1. J Neurol. 2023. PMID: 36048265 Free PMC article.
-
COVID-19 infection and vaccination in immunodeficiency.Clin Exp Immunol. 2022 Sep 29;209(3):259-261. doi: 10.1093/cei/uxac080. Clin Exp Immunol. 2022. PMID: 35972956 Free PMC article.
-
CD19 B cell repopulation after ocrelizumab, alemtuzumab and cladribine: Implications for SARS-CoV-2 vaccinations in multiple sclerosis.Mult Scler Relat Disord. 2022 Jan;57:103448. doi: 10.1016/j.msard.2021.103448. Epub 2021 Dec 4. Mult Scler Relat Disord. 2022. PMID: 34902760 Free PMC article.
-
Long-term immunological consequences of anti-CD20 therapies on humoral responses to COVID-19 vaccines in multiple sclerosis: an observational study.Ther Adv Neurol Disord. 2022 Apr 22;15:17562864221092092. doi: 10.1177/17562864221092092. eCollection 2022. Ther Adv Neurol Disord. 2022. PMID: 35479655 Free PMC article.
References
-
- Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun 2005, 8, 140–74. - PubMed
-
- VanDerMeid KR, Elliott MR, Baran AM, Barr PM, Chu CC, Zent CS. Cellular cytotoxicity of next-generation CD20 monoclonal antibodies. Cancer Immunol Res 2018, 6, 1150–60. - PubMed
-
- Baker D, Pryce G, Amor S, Giovannoni G, Schmierer K. Learning from other autoimmunities to understand targeting of B cells to control multiple sclerosis. Brain 2018, 141, 2834–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

