Mechanisms of ion transport regulation by HNF1β in the kidney: beyond transcriptional regulation of channels and transporters
- PMID: 35554666
- PMCID: PMC9338905
- DOI: 10.1007/s00424-022-02697-5
Mechanisms of ion transport regulation by HNF1β in the kidney: beyond transcriptional regulation of channels and transporters
Erratum in
-
Correction to: Mechanisms of ion transport regulation by HNF1β in the kidney: beyond transcriptional regulation of channels and transporters.Pflugers Arch. 2022 Aug;474(8):917. doi: 10.1007/s00424-022-02706-7. Pflugers Arch. 2022. PMID: 35608669 Free PMC article. No abstract available.
Abstract
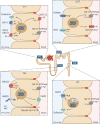
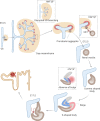
Hepatocyte nuclear factor 1β (HNF1β) is a transcription factor essential for the development and function of the kidney. Mutations in and deletions of HNF1β cause autosomal dominant tubule interstitial kidney disease (ADTKD) subtype HNF1β, which is characterized by renal cysts, diabetes, genital tract malformations, and neurodevelopmental disorders. Electrolyte disturbances including hypomagnesemia, hyperuricemia, and hypocalciuria are common in patients with ADTKD-HNF1β. Traditionally, these electrolyte disturbances have been attributed to HNF1β-mediated transcriptional regulation of gene networks involved in ion transport in the distal part of the nephron including FXYD2, CASR, KCNJ16, and FXR. In this review, we propose additional mechanisms that may contribute to the electrolyte disturbances observed in ADTKD-HNF1β patients. Firstly, kidney development is severely affected in Hnf1b-deficient mice. HNF1β is required for nephron segmentation, and the absence of the transcription factor results in rudimentary nephrons lacking mature proximal tubule, loop of Henle, and distal convoluted tubule cluster. In addition, HNF1β is proposed to be important for apical-basolateral polarity and tight junction integrity in the kidney. Interestingly, cilia formation is unaffected by Hnf1b defects in several models, despite the HNF1β-mediated transcriptional regulation of many ciliary genes. To what extent impaired nephron segmentation, apical-basolateral polarity, and cilia function contribute to electrolyte disturbances in HNF1β patients remains elusive. Systematic phenotyping of Hnf1b mouse models and the development of patient-specific kidney organoid models will be essential to advance future HNF1β research.
Keywords: Apical-basolateral polarity; Electrolyte disturbances; HNF1β; Kidney development; Transcriptional regulation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Aboudehen K, Kim MS, Mitsche M, Garland K, Anderson N, Noureddine L, Pontoglio M, Patel V, Xie Y, DeBose-Boyd R, Igarashi P. Transcription factor hepatocyte nuclear factor-1 regulates renal cholesterol metabolism. J Am Soc Nephrol. 2016;27:2408–2421. doi: 10.1681/ASN.2015060607. - DOI - PMC - PubMed
-
- Aboudehen K, Noureddine L, Cobo-Stark P, Avdulov S, Farahani S, Gearhart MD, Bichet DG, Pontoglio M, Patel V, Igarashi P. Hepatocyte nuclear factor–1 β regulates urinary concentration and response to hypertonicity. J Am Soc Nephrol. 2017;28:2887–2900. doi: 10.1681/ASN.2016101095. - DOI - PMC - PubMed
-
- Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, Hennekam RC, Ledermann SE, Rees L, Van HW, Marks SD, Trompeter RS, Tullus K, Winyard PJ, Cansick J, Mushtaq I, Dhillon HK, Bingham C, Edghill EL, Shroff R, Stanescu H, Ryffel GU. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol. 2009;20:1123–1131. doi: 10.1681/ASN.2008060633. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

