Oral eltrombopag versus subcutaneous recombinant human thrombopoietin for promoting platelet engraftment after allogeneic stem cell transplantation: A prospective, non-inferiority, randomized controlled trial
- PMID: 35554955
- PMCID: PMC9790607
- DOI: 10.1002/hon.3017
Oral eltrombopag versus subcutaneous recombinant human thrombopoietin for promoting platelet engraftment after allogeneic stem cell transplantation: A prospective, non-inferiority, randomized controlled trial
Abstract
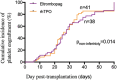
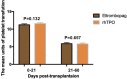
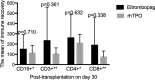
Delayed platelet engraftment (DPE) is associated with poor survival and increased transplantation-related mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Therefore, treatments are needed to improve platelet engraftment and prevent DPE. We performed a phase three, non-inferior, randomized controlled study of eltrombopag or recombinant human thrombopoietin (rhTPO) to promot platelet engraftment after allo-HSCT. Candidates for allo-HSCT were randomly assigned to receive oral eltrombopag (50 mg daily) or subcutaneous rhTPO (15000U daily) from the first-day post-transplantation. The primary endpoint was the cumulative numbers of platelet engraftment (platelet recovery ≥20 × 109 /L, without transfusion, for seven consecutive days) on day 60 after transplantation. We performed intention-to-treat analyses with a non-inferior margin of -15%. A total of 92 participants underwent randomization. 44 and 48 patients were randomized to the eltrombopag and rhTPO groups, respectively. The median duration of follow-up was 360 days (range: 12-960 days). The cumulative incidence of platelet engraftment on day 60 after transplantation in eltrombopag group was 86.4% (38/44) compared with 85.4% (41/48) in the rhTPO group (absolute risk difference [ARD] 1%, one-sided lower limit of 95% confidence interval [CI] -13.28%, Pnon-inferirioty = 0.014). The rate of DPE in the eltrombopag group was 6.8% (3/44) compared with 12.5% (6/48) in the rhTPO group (ARD -5.7%, one-sided higher limit of 95% CI 6.28%, Pnon-inferirioty = 0.063). Approximately, three-fourths of non-hematologic adverse events were not observed in the eltrombopag group but three patients (3/48, 6%) experienecd them in the rhTPO group. In addition, platelet transfusions unite from day 0 to day 21, or from day 22 to day 60, progression-free survival, overall survival were not significantly different between both groups. Eltrombopag was non-inferior to rhTPO in promoting platelet engraftment post allo-HSCT for patients with hematological malignancy. Oral eltrombopag was more convenient for patients than subcutaneous rhTPO (NCT03515096).
Keywords: allogeneic stem cell transplantation; eltrombopag; platelet engraftment; recombinant human thrombopoietin; rhTPO.
© 2022 The Authors. Hematological Oncology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Herombopag for the treatment of persistent thrombocytopenia following hematopoietic stem cell transplantation.Ann Hematol. 2024 May;103(5):1697-1704. doi: 10.1007/s00277-024-05711-1. Epub 2024 Mar 27. Ann Hematol. 2024. PMID: 38536476 Review.
-
Recombinant human thrombopoietin promotes platelet engraftment after haploidentical hematopoietic stem cell transplantation: a prospective randomized controlled trial.Ann Hematol. 2015 Jan;94(1):117-28. doi: 10.1007/s00277-014-2158-1. Epub 2014 Jul 30. Ann Hematol. 2015. PMID: 25069650 Clinical Trial.
-
Effect and safety of recombinant human thrombopoietin on haematopoietic reconstitution after allogeneic haematopoietic cell transplantation.Curr Res Transl Med. 2025 Jan-Mar;73(1):103472. doi: 10.1016/j.retram.2024.103472. Epub 2024 Sep 25. Curr Res Transl Med. 2025. PMID: 39368242
-
Recombinant Human Thrombopoietin Promotes Platelet Engraftment in Severe Aplastic Anemia Patients Following Treatment With Haploid Hematopoietic Stem Cell Transplantation using Modified Post-Transplantation Cyclophosphamide.Transplant Cell Ther. 2024 May;30(5):500-509. doi: 10.1016/j.jtct.2024.02.023. Epub 2024 Mar 4. Transplant Cell Ther. 2024. PMID: 38447750
-
Efficacy and safety of thrombopoietin receptor agonists in the treatment of thrombocytopenia after hematopoietic stem cell transplantation: a meta-analysis and systematic review.Expert Rev Hematol. 2021 Nov;14(11):1041-1048. doi: 10.1080/17474086.2021.2009337. Epub 2021 Dec 1. Expert Rev Hematol. 2021. PMID: 34844489
Cited by
-
Avatrombopag for the treatment of thrombocytopenia in children's patients following allogeneic hematopoietic stem-cell transplantation: A pilot study.Front Pediatr. 2023 Feb 15;11:1099372. doi: 10.3389/fped.2023.1099372. eCollection 2023. Front Pediatr. 2023. PMID: 36873638 Free PMC article.
-
Efficacy and safety of hetrombopag in the treatment of recombinant human thrombopoietin-resistant thrombocytopenia after allogeneic hematopoietic stem cell transplantation.Res Pract Thromb Haemost. 2024 Oct 9;8(7):102578. doi: 10.1016/j.rpth.2024.102578. eCollection 2024 Oct. Res Pract Thromb Haemost. 2024. PMID: 39628651 Free PMC article.
-
Safety of romiplostim administered immediately after cord-blood transplantation: a phase 1 trial.Ann Hematol. 2023 Oct;102(10):2895-2902. doi: 10.1007/s00277-023-05410-3. Epub 2023 Aug 17. Ann Hematol. 2023. PMID: 37589942 Clinical Trial.
-
Herombopag for the treatment of persistent thrombocytopenia following hematopoietic stem cell transplantation.Ann Hematol. 2024 May;103(5):1697-1704. doi: 10.1007/s00277-024-05711-1. Epub 2024 Mar 27. Ann Hematol. 2024. PMID: 38536476 Review.
-
Thrombopoietin Receptor Agonists in Post-Hematopoietic Cell Transplantation Complicated by Prolonged Thrombocytopenia: A Comprehensive Review.Immunotargets Ther. 2024 Sep 13;13:461-486. doi: 10.2147/ITT.S463384. eCollection 2024. Immunotargets Ther. 2024. PMID: 39290805 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

