Maturation Delay of Human GABAergic Neurogenesis in Fragile X Syndrome Pluripotent Stem Cells
- PMID: 35556144
- PMCID: PMC9216490
- DOI: 10.1093/stcltm/szac022
Maturation Delay of Human GABAergic Neurogenesis in Fragile X Syndrome Pluripotent Stem Cells
Abstract
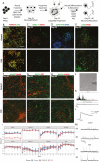
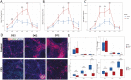
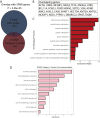
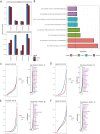
Fragile X Syndrome (FXS), the leading monogenic cause of intellectual disability and autism spectrum disorder, is caused by expansion of a CGG trinucleotide repeat in the 5'-UTR of the Fragile X Mental Retardation-1 (FMR1) gene. Epigenetic silencing of FMR1 results in loss of the Fragile X Mental Retardation Protein (FMRP). Although most studies to date have focused on excitatory neurons, recent evidence suggests that GABAergic inhibitory networks are also affected. To investigate human GABAergic neurogenesis, we established a method to reproducibly derive inhibitory neurons from multiple FXS and control human pluripotent stem cell (hPSC) lines. Electrophysiological analyses suggested that the developing FXS neurons had a delay in the GABA functional switch, a transition in fetal development that converts the GABAA channel's function from depolarization to hyperpolarization, with profound effects on the developing brain. To investigate the cause of this delay, we analyzed 14 400 single-cell transcriptomes from FXS and control cells at 2 stages of GABAergic neurogenesis. While control and FXS cells were similar at the earlier time point, the later-stage FXS cells retained expression of neuroblast proliferation-associated genes and had lower levels of genes associated with action potential regulation, synapses, and mitochondria compared with controls. Our analysis suggests that loss of FMRP prolongs the proliferative stage of progenitors, which may result in more neurons remaining immature during the later stages of neurogenesis. This could have profound implications for homeostatic excitatory-inhibitory circuit development in FXS, and suggests a novel direction for understanding disease mechanisms that may help to guide therapeutic interventions.
Keywords: GABAergic neurons; autism; fragile X syndrome; hESCs; human iPSCs; neurogenesis.
© The Author(s) 2022. Published by Oxford University Press.
Figures




Similar articles
-
Epigenetic characterization of the FMR1 gene and aberrant neurodevelopment in human induced pluripotent stem cell models of fragile X syndrome.PLoS One. 2011;6(10):e26203. doi: 10.1371/journal.pone.0026203. Epub 2011 Oct 12. PLoS One. 2011. PMID: 22022567 Free PMC article.
-
Human pluripotent stem cell models of Fragile X syndrome.Mol Cell Neurosci. 2016 Jun;73:43-51. doi: 10.1016/j.mcn.2015.11.011. Epub 2015 Nov 27. Mol Cell Neurosci. 2016. PMID: 26640241 Free PMC article. Review.
-
Epigenetic modifications in human fragile X pluripotent stem cells; Implications in fragile X syndrome modeling.Brain Res. 2017 Feb 1;1656:55-62. doi: 10.1016/j.brainres.2015.10.004. Epub 2015 Oct 20. Brain Res. 2017. PMID: 26475977 Review.
-
CGG-repeat dynamics and FMR1 gene silencing in fragile X syndrome stem cells and stem cell-derived neurons.Mol Autism. 2016 Oct 6;7:42. doi: 10.1186/s13229-016-0105-9. eCollection 2016. Mol Autism. 2016. PMID: 27713816 Free PMC article.
-
Development of GABAergic Inputs Is Not Altered in Early Maturation of Adult Born Dentate Granule Neurons in Fragile X Mice.eNeuro. 2018 Dec 3;5(6):ENEURO.0137-18.2018. doi: 10.1523/ENEURO.0137-18.2018. eCollection 2018 Nov-Dec. eNeuro. 2018. PMID: 30627633 Free PMC article.
Cited by
-
Mitochondrial metabolism in neural stem cells and implications for neurodevelopmental and neurodegenerative diseases.J Transl Med. 2024 Mar 4;22(1):238. doi: 10.1186/s12967-024-05041-w. J Transl Med. 2024. PMID: 38438847 Free PMC article. Review.
-
SLC35A2 loss of function variants affect glycomic signatures, neuronal fate, and network dynamics.bioRxiv [Preprint]. 2024 Dec 27:2024.12.27.630524. doi: 10.1101/2024.12.27.630524. bioRxiv. 2024. Update in: Brain. 2025 May 26:awaf198. doi: 10.1093/brain/awaf198. PMID: 39763953 Free PMC article. Updated. Preprint.
-
Fmrp regulates neuronal balance in embryonic motor circuit formation.Front Neurosci. 2022 Nov 3;16:962901. doi: 10.3389/fnins.2022.962901. eCollection 2022. Front Neurosci. 2022. PMID: 36408418 Free PMC article.
-
Harnessing the potential of human induced pluripotent stem cells, functional assays and machine learning for neurodevelopmental disorders.Front Neurosci. 2025 Jan 8;18:1524577. doi: 10.3389/fnins.2024.1524577. eCollection 2024. Front Neurosci. 2025. PMID: 39844857 Free PMC article. Review.
-
Fragile X cortex is characterized by decreased parvalbumin-expressing interneurons.Cereb Cortex. 2024 Mar 1;34(3):bhae103. doi: 10.1093/cercor/bhae103. Cereb Cortex. 2024. PMID: 38521994 Free PMC article.
References
-
- Cornish K, Turk J, Hagerman R.. The fragile X continuum: new advances and perspectives. J Intellect Disabil Res. 2008;52(Pt 6):469-482. - PubMed
-
- Lewis P, Abbeduto L, Murphy M, et al. . Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. J Intellect Disabil Res. 2006;50(Pt 7):532-545. - PubMed
-
- Pasciuto E, Bagni C.. SnapShot: FMRP interacting proteins. Cell 2014;159(1):218-2e1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

