Paradigm shift: new concepts for HCN4 function in cardiac pacemaking
- PMID: 35556164
- PMCID: PMC9192375
- DOI: 10.1007/s00424-022-02698-4
Paradigm shift: new concepts for HCN4 function in cardiac pacemaking
Abstract
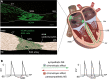
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels are the molecular correlate of the If current and are critically involved in controlling neuronal excitability and the autonomous rhythm of the heart. The HCN4 isoform is the main HCN channel subtype expressed in the sinoatrial node (SAN), a tissue composed of specialized pacemaker cells responsible for generating the intrinsic heartbeat. More than 40 years ago, the If current was first discovered in rabbit SAN tissue. Along with this discovery, a theory was proposed that cyclic adenosine monophosphate-dependent modulation of If mediates heart rate regulation by the autonomic nervous system-a process called chronotropic effect. However, up to the present day, this classical theory could not be reliably validated. Recently, new concepts emerged confirming that HCN4 channels indeed play an important role in heart rate regulation. However, the cellular mechanism by which HCN4 controls heart rate turned out to be completely different than originally postulated. Here, we review the latest findings regarding the physiological role of HCN4 in the SAN. We describe a newly discovered mechanism underlying heart rate regulation by HCN4 at the tissue and single cell levels, and we discuss these observations in the context of results from previously studied HCN4 mouse models.
Keywords: Autonomic nervous system; Chronotropic effect; HCN4 channel; Heart rate regulation; Hyperpolarization-activated cyclic nucleotide–gated channels; Sinoatrial node (SAN).
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Baruscotti M, Bucchi A, Viscomi C, Mandelli G, Consalez G, Gnecchi-Rusconi T, Montano N, Casali KR, Micheloni S, Barbuti A, DiFrancesco D. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc Natl Acad Sci U S A. 2011;108(4):1705–1710. doi: 10.1073/pnas.1010122108. - DOI - PMC - PubMed
-
- Baudot M, Torre E, Bidaud I, Louradour J, Torrente AG, Fossier L, Talssi L, Nargeot J, Barrere-Lemaire S, Mesirca P, Mangoni ME. Concomitant genetic ablation of L-type Cav1.3 (alpha1D) and T-type Cav3.1 (alpha1G) Ca(2+) channels disrupts heart automaticity. Sci Rep. 2020;10(1):18906. doi: 10.1038/s41598-020-76049-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

