TNF-α Orchestrates Experience-Dependent Plasticity of Excitatory and Inhibitory Synapses in the Anterior Piriform Cortex
- PMID: 35557610
- PMCID: PMC9086849
- DOI: 10.3389/fnins.2022.824454
TNF-α Orchestrates Experience-Dependent Plasticity of Excitatory and Inhibitory Synapses in the Anterior Piriform Cortex
Abstract
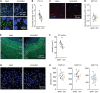
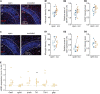
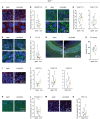
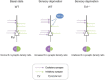
Homeostatic synaptic plasticity, which induces compensatory modulation of synapses, plays a critical role in maintaining neuronal circuit function in response to changing activity patterns. Activity in the anterior piriform cortex (APC) is largely driven by ipsilateral neural activity from the olfactory bulb and is a suitable system for examining the effects of sensory experience on cortical circuits. Pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) can modulate excitatory and inhibitory synapses, but its role in APC is unexplored. Here we examined the role of TNF-α in adjusting synapses in the mouse APC after experience deprivation via unilateral naris occlusion. Immunofluorescent staining revealed that activity deprivation increased excitatory, and decreased inhibitory, synaptic density in wild-type mice, consistent with homeostatic regulation. Quantitative RT-PCR showed that naris occlusion increased the expression of Tnf mRNA in APC. Critically, occlusion-induced plasticity of excitatory and inhibitory synapses was completely blocked in the Tnf knockout mouse. Together, these results show that TNF-α is an important orchestrator of experience-dependent plasticity in the APC.
Keywords: GABAergic interneurons; cytokines; excitation and inhibition balance; glial cells; homeostatic plasticity.
Copyright © 2022 Guo and Lau.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Retinoic Acid Receptor RARα-Dependent Synaptic Signaling Mediates Homeostatic Synaptic Plasticity at the Inhibitory Synapses of Mouse Visual Cortex.J Neurosci. 2018 Dec 5;38(49):10454-10466. doi: 10.1523/JNEUROSCI.1133-18.2018. Epub 2018 Oct 24. J Neurosci. 2018. PMID: 30355624 Free PMC article.
-
Postnatal development of inhibitory synaptic transmission in the anterior piriform cortex.Int J Dev Neurosci. 2018 Dec;71:1-9. doi: 10.1016/j.ijdevneu.2018.07.008. Epub 2018 Jul 25. Int J Dev Neurosci. 2018. PMID: 30055229
-
Disruption of NMDAR Function Prevents Normal Experience-Dependent Homeostatic Synaptic Plasticity in Mouse Primary Visual Cortex.J Neurosci. 2019 Sep 25;39(39):7664-7673. doi: 10.1523/JNEUROSCI.2117-18.2019. Epub 2019 Aug 14. J Neurosci. 2019. PMID: 31413075 Free PMC article.
-
The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits.Prog Brain Res. 2008;169:211-23. doi: 10.1016/S0079-6123(07)00012-X. Prog Brain Res. 2008. PMID: 18394476 Review.
-
Synaptic Plasticity in Cortical Inhibitory Neurons: What Mechanisms May Help to Balance Synaptic Weight Changes?Front Cell Neurosci. 2020 Sep 4;14:204. doi: 10.3389/fncel.2020.00204. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33100968 Free PMC article. Review.
Cited by
-
The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective.Life (Basel). 2025 May 14;15(5):785. doi: 10.3390/life15050785. Life (Basel). 2025. PMID: 40430212 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

