Sensitivity of Diffusion MRI to White Matter Pathology: Influence of Diffusion Protocol, Magnetic Field Strength, and Processing Pipeline in Systemic Lupus Erythematosus
- PMID: 35557624
- PMCID: PMC9087851
- DOI: 10.3389/fneur.2022.837385
Sensitivity of Diffusion MRI to White Matter Pathology: Influence of Diffusion Protocol, Magnetic Field Strength, and Processing Pipeline in Systemic Lupus Erythematosus
Abstract
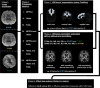
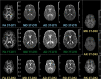
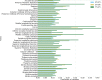
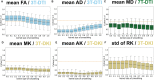
There are many ways to acquire and process diffusion MRI (dMRI) data for group studies, but it is unknown which maximizes the sensitivity to white matter (WM) pathology. Inspired by this question, we analyzed data acquired for diffusion tensor imaging (DTI) and diffusion kurtosis imaging (DKI) at 3T (3T-DTI and 3T-DKI) and DTI at 7T in patients with systemic lupus erythematosus (SLE) and healthy controls (HC). Parameter estimates in 72 WM tracts were obtained using TractSeg. The impact on the sensitivity to WM pathology was evaluated for the diffusion protocol, the magnetic field strength, and the processing pipeline. Sensitivity was quantified in terms of Cohen's d for group comparison. Results showed that the choice of diffusion protocol had the largest impact on the effect size. The effect size in fractional anisotropy (FA) across all WM tracts was 0.26 higher when derived by DTI than by DKI and 0.20 higher in 3T compared with 7T. The difference due to the diffusion protocol was larger than the difference due to magnetic field strength for the majority of diffusion parameters. In contrast, the difference between including or excluding different processing steps was near negligible, except for the correction of distortions from eddy currents and motion which had a clearly positive impact. For example, effect sizes increased on average by 0.07 by including motion and eddy correction for FA derived from 3T-DTI. Effect sizes were slightly reduced by the incorporation of denoising and Gibbs-ringing removal (on average by 0.011 and 0.005, respectively). Smoothing prior to diffusion model fitting generally reduced effect sizes. In summary, 3T-DTI in combination with eddy current and motion correction yielded the highest sensitivity to WM pathology in patients with SLE. However, our results also indicated that the 3T-DKI and 7T-DTI protocols used here may be adjusted to increase effect sizes.
Keywords: DKI; DTI; ROI-based analysis; diffusion MRI; diffusion processing; effect sizes; ultra-high magnetic field strength (7T); white matter fiber-tracts.
Copyright © 2022 Kornaropoulos, Winzeck, Rumetshofer, Wikstrom, Knutsson, Correia, Sundgren and Nilsson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


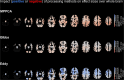



Similar articles
-
Differences in Gaussian diffusion tensor imaging and non-Gaussian diffusion kurtosis imaging model-based estimates of diffusion tensor invariants in the human brain.Med Phys. 2016 May;43(5):2464. doi: 10.1118/1.4946819. Med Phys. 2016. PMID: 27147357
-
White matter tracts in Bipolar Disorder patients: A comparative study based on diffusion kurtosis and tensor imaging.J Affect Disord. 2021 Sep 1;292:45-55. doi: 10.1016/j.jad.2021.05.030. Epub 2021 May 27. J Affect Disord. 2021. PMID: 34098469
-
The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review.Neurosurg Focus. 2016 Sep;41(3):E12. doi: 10.3171/2016.6.FOCUS16192. Neurosurg Focus. 2016. PMID: 27581308 Review.
-
High-resolution diffusion kurtosis imaging at 3T enabled by advanced post-processing.Front Neurosci. 2015 Jan 7;8:427. doi: 10.3389/fnins.2014.00427. eCollection 2014. Front Neurosci. 2015. PMID: 25620906 Free PMC article.
-
Assessing White Matter Pathology in Early-Stage Parkinson Disease Using Diffusion MRI: A Systematic Review.Front Neurol. 2020 May 14;11:314. doi: 10.3389/fneur.2020.00314. eCollection 2020. Front Neurol. 2020. PMID: 32477235 Free PMC article.
Cited by
-
Denoising Improves Cross-Scanner and Cross-Protocol Test-Retest Reproducibility of Diffusion Tensor and Kurtosis Imaging.Hum Brain Mapp. 2025 Mar;46(4):e70142. doi: 10.1002/hbm.70142. Hum Brain Mapp. 2025. PMID: 40051327 Free PMC article.
-
Optimization and validation of the DESIGNER preprocessing pipeline for clinical diffusion MRI in white matter aging.Imaging Neurosci (Camb). 2024 Apr 8;2:imag-2-00125. doi: 10.1162/imag_a_00125. eCollection 2024. Imaging Neurosci (Camb). 2024. PMID: 40800272 Free PMC article.
-
Impact of truncating diffusion MRI scans on diffusional kurtosis imaging.MAGMA. 2024 Oct;37(5):859-872. doi: 10.1007/s10334-024-01153-y. Epub 2024 Feb 23. MAGMA. 2024. PMID: 38393541 Free PMC article.
-
Topological structural characteristics in patients with systemic lupus erythematosus with and without neuropsychiatric symptoms.Lupus Sci Med. 2024 Sep 11;11(2):e001221. doi: 10.1136/lupus-2024-001221. Lupus Sci Med. 2024. PMID: 39266226 Free PMC article.
-
Comparison of distortion correction preprocessing pipelines for DTI in the upper limb.Magn Reson Med. 2024 Feb;91(2):773-783. doi: 10.1002/mrm.29881. Epub 2023 Oct 13. Magn Reson Med. 2024. PMID: 37831659 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

