Mercury Removal from Water Using a Novel Composite of Polyacrylate-Modified Carbon
- PMID: 35557665
- PMCID: PMC9088914
- DOI: 10.1021/acsomega.2c00274
Mercury Removal from Water Using a Novel Composite of Polyacrylate-Modified Carbon
Abstract
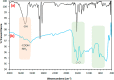
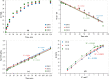
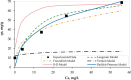
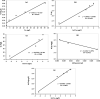
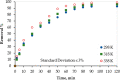
The contamination of groundwater by mercury (Hg) is a serious global threat, and its removal is of great importance. Activated carbon (AC) is considered a very promising adsorbent to remove Hg from water systems. However, specific functional groups can be added to AC to enhance its adsorption efficiency. In this work, AC was synthesized from palm shells and grafted with a copolymer of acrylamide and methacrylic acid to produce a polyacrylate-modified carbon (PAMC) composite. The synthesized adsorbent (PAMC) was characterized by Fourier-transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), electron dispersive X-ray (EDX) spectroscopy, and Brunauer-Emmett-Teller (BET) analysis. PAMC was then evaluated for Hg removal from aqueous solutions, and the adsorption efficiency was optimized under several parameters (pH, contact time, and PAMC dosage). Kinetic, isotherm, and thermodynamic investigations were performed to gain a further understanding of the adsorption properties. The adsorption data were best fitted by pseudo-second-order and Redlich-Peterson models. Also, the thermodynamic investigation confirmed the spontaneity and the endothermic nature of the Hg adsorption process over PAMC. The maximum adsorption capacity (q m) of PAMC was found to be 76.3 mg/g ,which is relatively higher than some activated carbon-based adsorbents. Therefore, PAMC offers a potential promise for wastewater treatment due to its fast and high uptake removal capacity in addition to the cheap and environmentally friendly activated carbon source.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Zheng H.; Hong J.; Luo X.; Li S.; Wang M.; Yang B.; Wang M. Combination of sequential cloud point extraction and hydride generation atomic fluorescence spectrometry for preconcentration and determination of inorganic and methyl mercury in water samples. Microchem. J. 2019, 145, 806–812. 10.1016/j.microc.2018.11.057. - DOI
-
- Altunay N. Utility of ultrasound assisted-cloud point extraction and spectrophotometry as a preconcentration and determination tool for the sensitive quantification of mercury species in fish samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 189, 167–175. 10.1016/j.saa.2017.08.033. - DOI - PubMed
LinkOut - more resources
Full Text Sources

