Solid-State Luminescent Materials Containing Both Indole and Pyrimidine Moieties: Design, Synthesis, and Density Functional Theory Calculations
- PMID: 35557695
- PMCID: PMC9089344
- DOI: 10.1021/acsomega.2c00775
Solid-State Luminescent Materials Containing Both Indole and Pyrimidine Moieties: Design, Synthesis, and Density Functional Theory Calculations
Abstract
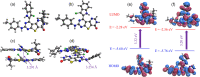
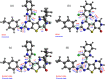
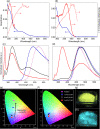
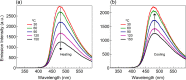
Heterocyclic compounds with effective solid-state luminescence offer a wide range of uses. It has been observed that combining pyrimidine and indole moieties in a single molecule can enhance material behavior dramatically. Here, different heterocyclic compounds with indole and pyrimidine moieties have been synthesized effectively, and their structures have been validated using NMR, IR, and mass spectroscopy. The photoluminescence behavior of two substances was investigated in powder form and solutions of varying concentrations. After aggregation, one molecule displayed a redshifted luminescence spectrum, whereas another homolog showed a blueshift. Thus, density functional theory calculations were carried out to establish that introducing a terminal group allows modifying of the luminescence behavior by altering the molecular packing. Because of the non-planarity, intermolecular interactions, and tiny intermolecular distances within the dimers, the materials demonstrated a good emission quantum yield (Φem) in the solid state (ex. 25.6%). At high temperatures, the compounds also demonstrated a stable emission characteristic.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Tang C. W.; Vanslyke S. A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913–915. 10.1063/1.98799. - DOI
LinkOut - more resources
Full Text Sources

