Mechanistic Insight into the Precursor Chemistry of ZrO2 and HfO2 Nanocrystals; towards Size-Tunable Syntheses
- PMID: 35557760
- PMCID: PMC9088301
- DOI: 10.1021/jacsau.1c00568
Mechanistic Insight into the Precursor Chemistry of ZrO2 and HfO2 Nanocrystals; towards Size-Tunable Syntheses
Erratum in
-
Correction to "Mechanistic Insight into the Precursor Chemistry of ZrO2 and HfO2 Nanocrystals, toward Size-Tunable Syntheses".JACS Au. 2022 Apr 28;2(5):1232. doi: 10.1021/jacsau.2c00231. eCollection 2022 May 23. JACS Au. 2022. PMID: 35647597 Free PMC article.
Abstract
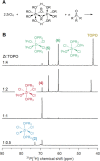
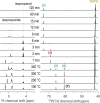
One can nowadays readily generate monodisperse colloidal nanocrystals, but a retrosynthetic analysis is still not possible since the underlying chemistry is often poorly understood. Here, we provide insight into the reaction mechanism of colloidal zirconia and hafnia nanocrystals synthesized from metal chloride and metal isopropoxide. We identify the active precursor species in the reaction mixture through a combination of nuclear magnetic resonance spectroscopy (NMR), density functional theory (DFT) calculations, and pair distribution function (PDF) analysis. We gain insight into the interaction of the surfactant, tri-n-octylphosphine oxide (TOPO), and the different precursors. Interestingly, we identify a peculiar X-type ligand redistribution mechanism that can be steered by the relative amount of Lewis base (L-type). We further monitor how the reaction mixture decomposes using solution NMR and gas chromatography, and we find that ZrCl4 is formed as a by-product of the reaction, limiting the reaction yield. The reaction proceeds via two competing mechanisms: E1 elimination (dominating) and SN1 substitution (minor). Using this new mechanistic insight, we adapted the synthesis to optimize the yield and gain control over nanocrystal size. These insights will allow the rational design and synthesis of complex oxide nanocrystals.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Park Y. M.; Desai A.; Salleo A.; Jimison L. Solution-Processable Zirconium Oxide Gate Dielectrics for Flexible Organic Field Effect Transistors Operated at Low Voltages. Chem. Mater. 2013, 25, 2571–2579. 10.1021/cm303547a. - DOI
-
- Chang J. P.; Lin Y. S.; Berger S.; Kepten A.; Bloom R.; Levy S. Ultrathin zirconium oxide films as alternative gate dielectrics. J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct. 2001, 19, 2137.10.1116/1.1415513. - DOI
-
- Rijckaert H.; Pollefeyt G.; Sieger M.; Hänisch J.; Bennewitz J.; De Keukeleere K.; De Roo J.; Hühne R.; Bäcker M.; Paturi P.; Huhtinen H.; Hemgesberg M.; Van Driessche I. Optimizing Nanocomposites through Nanocrystal Surface Chemistry: Superconducting YBa2Cu3O7 Thin Films via Low-Fluorine Metal Organic Deposition and Preformed Metal Oxide Nanocrystals. Chem. Mater. 2017, 29, 6104–6113. 10.1021/acs.chemmater.7b02116. - DOI
LinkOut - more resources
Full Text Sources
