Schiff base complex conjugates of bovine serum albumin as artificial metalloenzymes for eco-friendly enantioselective sulfoxidation
- PMID: 35557885
- PMCID: PMC9091609
- DOI: 10.1039/c8ra07113f
Schiff base complex conjugates of bovine serum albumin as artificial metalloenzymes for eco-friendly enantioselective sulfoxidation
Abstract
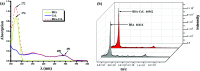
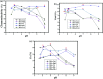
Artificial metalloenzymes (BSA-ML) have been prepared by non-covalent insertion of transition metal Schiff-base complexes, ML (L = 2-hydroxynaphthalen-1-naphthaldehyde and 3,4-diaminobenzenesulfonic acid; M = Co, Mn, V, Fe, Cr), into bovine serum albumin (BSA) as the host protein and were characterized by UV-visible spectroscopy, ESI-TOF mass spectrometry and molecular docking studies. The catalytic activities of the BSA-ML in the selective oxidation of various prochiral sulfides in aqueous media, using H2O2 as oxidant, have been evaluated. During the optimization process, pH and the concentrations of catalyst and oxidant were found to have a remarkable influence on both yield and enantioselectivity. In certain cases, BSA-ML gave satisfactory results in the oxidation of organic sulfides to sulfoxides (up to 100% conversion, 100% chemoselectivity, 96% ee and 500 h-1 turnover frequency).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

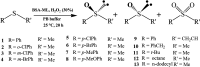

References
-
- Zhang Z.-H. Yang X.-S. Zhang Q.-Q. Wang L. He M.-Y. Chen Q. Huang X.-F. RSC Adv. 2016;6:104036–104040. doi: 10.1039/C6RA22393A. - DOI
-
- Pires S. M. G. Simões M. M. Q. Santos I. C. M. S. Rebelo S. L. H. Pereira M. M. Neves M. G. P. M. S. Cavaleiro J. A. S. Appl. Catal., A. 2012;439–440:51–56. doi: 10.1016/j.apcata.2012.06.044. - DOI
Publication types
LinkOut - more resources
Full Text Sources

